
Usually, these and the other metals are divided into several subgroups for specific purposes:
- Actinides - group 3: actinium (Ac) through lawrencium (Lr).
- Alkali metals - group 1: lithium (Li) through francium (Fr).
- Alkaline earth metals - group 2: beryllium (Be) through radium (Ra).
- Aluminides - aluminum (Al): native aluminum and minerals above 25 at % Al.
- Body-centered cubic metals - titanium (Ti) through chromium (Cr), zirconium (Zr) through molybdenum (Mo), and hafnium (Hf) through tungsten (W).
- Heavy metals - mercury (Hg) through polonium (Po), copernicium (Cn) through livermorium (Lv).
- Lanthanides - lanthanum (La) through lutetium (Lu), also called the rare-earths.
- Metalloids - gallium (Ga) through selenium (Se) and cadmium (Cd) through tellurium (Te).
- Precious metals - technetium (Tc) through silver (Ag) and rhenium (Re) through gold (Au).
- Rare earth metals group 3: scandium (Sc), yttrium (Y), the Lanthanides, and the Actinides.
- Siliconides - native silicon and minerals above 25 at % Si.
- Transition metals are often restricted to manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn), but generally include Sc through zinc (Zn), Y through cadmium (Cd), lanthanum through mercury, actinium through copernicium (Cn).
- Transuranics - neptunium (Np) through roentgenium (Rg) and copernicium (Cn).
For this lecture, the transition metals are limited to those minerals containing significant quantities of manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn).
Manganeses
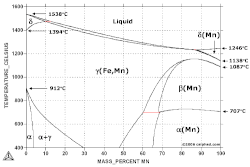
If native manganese occurs on Earth or nearby Solar System bodies, it likely occurs as bcc α-Mn.
"Beta manganese has a cubic crystal structure with space group P4132 [1]. The unit cell contains 20 atoms, divided between two non-equivalent sites."[1]
"The structures of γ- and δ-manganese are found to be face-centred cubic and body-centred cubic respectively."[2]
Irons

.png.webp)
The polished piece on the right displays inclusions of native iron.
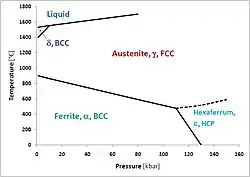
Iron occurs in several allotropes from α-Fe which has a body-centered cubic structure (bcc) at room temperature up to 910°C, γ-Fe which has a face-centered cubic (fcc) structure from 910°C to 1394°C, and δ-Fe (bcc) from 1394°C to 1538°C. Hexagonal close-packed (hcp) iron occurs at high pressures and temperatures as ε-Fe.
Kamacites

"Kamacite is an alloy of iron and nickel, which is only found on earth in meteorites. The proportion iron:nickel is between 90:10 to 95:5; small quantities of other elements, such as cobalt or carbon may also be present. The mineral has a metallic luster, is gray and has no clear cleavage although the structure is isometric-hexoctahedral. Its density is around 8 g/cm³ and its hardness is 4 on the Mohs scale. It is also sometimes called balkeneisen."[3]
Taenites
"Taenite (Fe,Ni) is a mineral found naturally on Earth mostly in iron meteorites. It is an alloy of iron and nickel, with[nickel proportions of 20% up to 65%. ... Taenite is one of four known Fe-Ni meteorite minerals: The others are kamacite, tetrataenite, and antitaenite. ... It is opaque with a metallic grayish to white color. The structure is isometric-hexoctahedral. Its density is around 8 g/cm³ and hardness is 5 to 5.5 on the Mohs scale. Taenite is magnetic. The crystal lattice has the c≈a= 3.582ű0.002Å.[4] The Strunz classification is I/A.08-20, while the Dana classification is 1.1.11.2 . It is a Hexoctahedral (cubic) in structure."[5]
Tetrataenites
"Tetrataenite is a native metal found in meteorites with the composition FeNi. It is one of the mineral phases found in meteoric iron.[6][7][8]"[9]
Antitaenites
"Antitaenite is a meteoritic metal alloy mineral composed of iron and nickel, 20-40% Ni (and traces of other elements) that has a face centered cubic crystal structure. ... [It exists] as a new mineral species occurring in both iron meteorites and in chondrites[10] ... The pair of minerals antitaenite and taenite constitute the first example in nature of two minerals that have the same crystal structure (face centered cubic) and can have the same chemical composition (same proportions of Fe and Ni) - but differ in their electronic structures: taenite has a high magnetic moment whereas antitaenite has a low magnetic moment.[11] [This difference] arises from a high-magnetic-moment to low-magnetic-moment transition occurring in the Fe-Ni bi-metallic alloy series.[12]"[13]
Cobalts
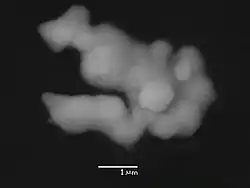
Cobalt has a hexagonal close-packed structure (hcp) until about 450°C when a fcc structure begins to appear.
On the right is a scanning electron micrograph of native cobalt from the Luna 24 landing site, Mare Crisium, The Moon.
Nickels
.jpg.webp)
{{free media}}Niccolite has the chemical formula NiAs.[14]
Coppers

The most advantageous form for copper is native copper.
On the right is a large, sculptural specimen of penny-bright copper from Arizona.
Cuprites

Cuprite (Cu2) is an oxide of copper that is 66.7 at % copper. It occurs "often as an important ore mineral, in the oxidized zone of copper deposits."[14]
It is a cubic mineral of space group Pn3m with Z=2 (two formula or molecular units per unit cell.
Zincs

{{free media}}Smithsonite has the chemical formula ZnCO
3.[14]
Hypotheses
- Transition metals are the border between fissionable and fusionable elements.
See also
- Chemicals/Cobalts
- Chemicals/Coppers
- Chemicals/Irons
- Chemicals/Manganeses
- Chemicals/Nickels
- Metal minerals
References
- ↑ J.B. Dunlop; J.M. Williams; J. Crangle (January-March 1977). "119Sn Mössbauer and neutron diffraction investigation of β Mn-Sn solid solutions". Physica B+C 86-88: 269-71. doi:10.1016/0378-4363(77)90310-2. http://www.sciencedirect.com/science/article/pii/0378436377903102. Retrieved 2015-08-19.
- ↑ Z. S. Basinski; J. W. Christian (20 May 1954). "A Pressurized High-Temperature Debye-Scherrer Camera, and Its Use to Determine the Structures and Coefficients of Expansion of γ- and δ-manganese". The Royal Society Proceedings A 223 (1155): 554. doi:10.1098/rspa.1954.0136. http://rspa.royalsocietypublishing.org/content/223/1155/554.short. Retrieved 2015-08-19.
- ↑ "Kamacite". San Francisco, California: Wikimedia Foundation, Inc. August 4, 2013. Retrieved 2013-09-01.
- ↑ Albertsen, F.; Knudsen, J. M.; Jensen, G. B. (Jun). "Structure of taenite in two iron meteorites J.". Nature 273 (5662): 453–454. doi:10.1038/273453a0.
- ↑ "Taenite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. February 27, 2013. Retrieved 2013-09-01.
- ↑ "Tetrataenite". webmineral.com.
- ↑ Mindat.org - Tetrataenite
- ↑ Handbook of Mineralogy - Tetrataenite
- ↑ "Tetrataenite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. July 24, 2013. Retrieved 2013-09-01.
- ↑ D.G. Rancourt and R.B. Scorzelli. Low Spin γ-Fe-Ni (γLS) Proposed as a New Mineral in Fe-Ni-Bearing Meteorites: Epitaxial Intergrowth of γLS and Tetrataenite as Possible Equilibrium State at ~20-40 at % Ni. Journal of Magnetism and Magnetic Materials 150 (1995) 30-36
- ↑ D.G. Rancourt, K. Lagarec, A. Densmore, R.A. Dunlap, J.I. Goldstein, R.J. Reisener, and R.B. Scorzelli. Experimental Proof of the Distinct Electronic Structure of a New Meteoritic Fe-Ni Alloy Phase. Journal of Magnetism and Magnetic Materials 191 (1999) L255-L260
- ↑ K. Lagarec, D.G. Rancourt, S.K. Bose, B. Sanyal, and R.A. Dunlap. Observation of a composition-controlled high-moment/low-moment transition in the face centered cubic Fe-Ni system: Invar effect is an expansion, not a contraction. Journal of Magnetism and Magnetic Materials 236 (2001) 107-130.
- ↑ "Antitaenite, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. May 7, 2013. Retrieved 2013-09-01.
- 1 2 3 Willard Lincoln Roberts; George Robert Rapp Jr.; Julius Weber (1974). Encyclopedia of Minerals. New York, New York, USA: Van Nostrand Reinhold Company. pp. 121–2. ISBN 0-442-26820-3.