
The aluminides are those naturally occurring minerals with a high atomic % aluminum.
In the image on the right of a flake of native aluminum, the scale bar = 1 mm.
"Aluminium is the third most abundant element (after oxygen and silicon) in the Earth's crust, and the most abundant metal there. It makes up about 8% by mass of the crust, though it is less common in the mantle below."[1]
Native aluminums

The image at the top of this lecture is one of two images exhibiting native aluminum.
This flake was discovered, "During a field trip to the NW Rila Mountain in the early 1960s, one of us (V.A.) investigated the desilicated pegmatite apophysis and, from the phlogopite zone (Fig. 1c), collected a rock specimen with a protruding metallic flake visible to the naked eye (Fig. 2) [from which the above image was cropped]."[2]
The designation for native aluminum is Al0 as indicated in, "Here we present data for a unique Al0 flake protruding from the phlogopite matrix of a rock specimen collected from a desilicated pegmatite vein."[2]
The second image of native aluminum is shown on the right of this section. The sample is from a mud volcano in the Caspian Sea near Baku, Azerbaidzhan.
The type locality for native aluminum is the Tolbachik volcano, Kamchatka, Russia.
Corundums

Corundum is α-Al2O3. It has Z = 6 formula units per hexagonal unit cell.[3]
Corundum is 40 at % aluminum.
Gibbsites

Gibbsites have the chemical formula Al(OH)3 with eight formula units per monoclinic unit cell.[3]
Gibbsites have only about 14.3 at % aluminum.
Bayerites
Bayerite is a polymorph of gibbsite and has the same chemical formula. Part of the challenge of determining the unique structure of bayerite versus gibbsite is that bayerite appears to transform to gibbsite under certain circumstances. The structures may also interleave. The other two polymorphs: nordstrandite and doyleite, are also variations with possible interleaving.
Early structural determinations using powder diffraction studies appeared contradicting.
The first structural study (1942) found bayerite to be hexagonal with two formula units (Z) per unit cell.[4] The lattice parameters were a=5.01 Å and c=4.76 Å.[4]
A second study (1951) found bayerite to be monoclinic.[5]
A third study (1958) found bayerite to be hexagonal with a=5.047 Å and c=4.730 Å with Z=2.[6]
Doyleite "the mineral from Mont St. Hilaire [is] triclinic, space group P1̅ from morphology, a 5.002(1), b 5.175(1), c 4.980(2) , α 97.50(1), β 118.60(1), γ 104.74(1)°, Z = 2."[7]
Bayerite has a monoclinic structure and lattice parameters of a=5.062(1) Å, b=8.671(2) Å, c=4.713(1) Å, β =90.27(3)° with space group P21/a.[7]
"The primitive P1̅ unit cell of nordstrandite was confirmed to contain four formula units, unlike doyleite (Z = 2). The layered structures of nordstrandite and doyleite were shown to be closely related to that of bayerite, differing from one another by the interlayer shift vectors only."[8]
As of 2014, bayerite has the monoclinic (P21/m) (or brucite) structure.[9]
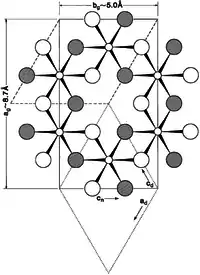
In the diagram on the right, an idealized "structure of gibbsite is projected on (001), showing geometric relations of the cells of gibbsite (solid lines), bayerite (dashed lines), doyleite (dotted lines) and nordstrandite. [Subscripts are] g for gibbsite, d for doyleite and n for nordstrandite. The oxygen atoms are at heights of 0.11 (shaded large circles) and -0.11 (unshaded)."[7]
Hypotheses
- In the case of native elements, proof of concept often consists of an actual specimen found in a natural setting with a composition that is similar to its setting rather than man-made artifacts of the same element.
See also
References
- ↑ "Aluminium". San Francisco, California: Wikimedia Foundation, Inc. 28 October 2015. Retrieved 2015-10-28.
- 1 2 Vesselin M. Dekov; Vasil Arnaudov; Frans Munnik; Tanya B. Boycheva; Saverio Fiore (2009). "Native aluminum: Does it exist?". American Mineralogist 94 (8-9): 1283-6. doi:10.2138/am.2009.3236. http://rruff.info/uploads/AM94_1283.pdf. Retrieved 2015-08-28.
- 1 2 Willard Lincoln Roberts; George Robert Rapp Jr.; Julius Weber (1974). Encyclopedia of Minerals. New York, New York, USA: Van Nostrand Reinhold Company. pp. 121–2. ISBN 0-442-26820-3. Retrieved 2015-07-30.
- 1 2 V. Montoro (1942). "Crystal Structure of Bayerite". Ricerca Sciencia 13: 565. https://www.jstage.jst.go.jp/article/bcsj1926/31/1/31_1_140/_article. Retrieved 2015-10-28.
- ↑ W. O. Milligan (1951). "Recent X-Ray Diffraction Studies on the Hydrous Oxides and Hydroxides". The Journal of Physical Chemistry 55 (4): 497-507. doi:10.1021/j150487a003. http://pubs.acs.org/doi/abs/10.1021/j150487a003. Retrieved 2015-10-28.
- ↑ Goro Yamaguchi; Kenichi Sakamoto (1958). "Crystal Structure of Bayerite". Bulletin of the Chemical Society of Japan 31 (1): 140-1. doi:10.1246/bcsj.31.140. https://www.jstage.jst.go.jp/article/bcsj1926/31/1/31_1_140/_article. Retrieved 2015-10-28.
- 1 2 3 George Y. Chao; Judith Baker; Ann P. Sabina; Andrew C. Roberts (1985). "Doyleite, A New Polymorph of Al(OH)3, and its Relationship to Bayerite, Gibbsite and Nordstrandite". Canadian Mineralogist 23: 21-8. http://rruff.info/doclib/cm/vol23/CM23_21.pdf. Retrieved 2015-10-28.
- ↑ Raffaella Demichelis; Michele Catti; Roberto Dovesi (2009). "Structure and stability of the Al (OH) 3 polymorphs doyleite and nordstrandite: a quantum mechanical ab initio study with the crystal06 code". The Journal of Physical Chemistry C 113 (16): 6785-91. doi:10.1021/jp810084c. http://pubs.acs.org/doi/abs/10.1021/jp810084c. Retrieved 2015-10-28.
- ↑ G.S.E. Antipas; N. Papassiopi; A. Xenidis (2014). "On the elusive anti-bayerite structure". Solid State Ionics 255: 65-73. doi:10.1016/j.ssi.2013.11.052. http://www.sciencedirect.com/science/article/pii/S0167273813006486. Retrieved 2015-10-28.