
The alkaline earth metals are the elements in Group 2 of the Periodic Table: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Their minerals usually contain more than 25 atomic % of these elements.
Berylliums
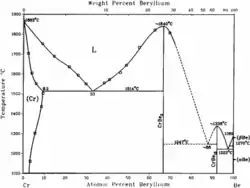
Beryllium occurs in a hexgonal close-packed (hcp) crystal structure at room temperature (α-Be).
As indicated in the phase diagram on the left beryllium occurs as (β-Be) which is bcc at higher temperatures up to melting.
Native beryllium is not known to occur on the surface of the Earth, but may eventually be found among beryllium-bearing minerals in small amounts.
Bromellites

{{free media}}Bromellite is BeO, with 50 at % beryllium.[1]
Magnesiums

Magnesium has a hcp structure from room temperature up to melting. No other phases occur as is shown in the magnesium-end of the iron-magnesium phase diagram on the left.
Native magnesium is unlikely to occur on the surface of the Earth and is not known to occur.
Chloromagnesites
Chloromagnesite, or chlormagnesite, has the formula MgCl2, with 33.3 at % magnesium.[1]
Forsterites

Mg2SiO4 is the formula for forsterite, with 28.6 at % magnesium.[1]
Forsterite is a member of the olivine group of minerals.[1]
Calciums

Calcium has a face-centered cubic (fcc) crystal structure at room temperature.
As shown in the phase diagram on the left, it does not change structure up to melting.
Native calcium is not known to occur on the surface of the Earth.
Fluorites

Fluorite has the formula CaF2 with 33.3 at % calcium.[1] Fluorites are also halogen minerals.
Bredigites
Bredigite is α-Ca2SiO4, with 28.5 at % calcium.[1]
γ-dicalcium silicates
γ-dicalcium silicate has the formula γ-Ca2SiO4, with 28.5 at % calcium.[1]
Strontiums
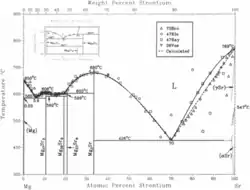
Strontium at room temperature crystallizes in a fcc structure (α-Sr).
According to the phase diagram on the left, α-Sr transforms to γ-Sr (bcc) at 547°C.
Native strontium does not appear to occur on the surface of the Earth.
Bariums
.png.webp)
Barium is bcc (α-Ba) at room temperature as the phase diagram on the left indicates. It does change to an hcp structure at high pressures and temperatures.
Native barium is not known to occur on the surface of the Earth.
Radiums
"Solid radium is bcc at room temperature. Radium melts at 973 K.63"[2]
Hypotheses
- Native alkaline earths may require very arid and reducing environments to occur.
See also
References
- 1 2 3 4 5 6 7 Willard Lincoln Roberts; George Robert Rapp Jr.; Julius Weber (1974). Encyclopedia of Minerals. New York, New York, USA: Van Nostrand Reinhold Company. p. 693. ISBN 0-442-26820-3.
- ↑ David A. Young (11 September 1975). Phase Diagrams of the Elements (PDF). University of California, Livermore, California USA: Lawrence Livermore Laboratory. p. 70. Retrieved 2015-08-26.