Absorption astronomy is the study of the various absorptions that occur during radiation or emissions from astronomical sources, or between sources and observational astronomy detectors.
Absorption can also be used by design in detectors.
Water ices
.jpg.webp)
Blue ice occurs when snow falls on a glacier, is compressed, and becomes part of a glacier. Blue ice was observed in Tasman Glacier, New Zealand in January 2011.[1] Ice is blue for the same reason water is blue: it is a result of an overtone of an oxygen-hydrogen (O-H) bond stretch in water which absorbs light at the red end of the visible spectrum.[2]
Cyan minerals
"The blue colors of several minerals and gems, including aquamarine (beryl, Be3Al2Si6O18) and cordierite (Al3(Mg, Fe)2Si5AlO18), have been attributed to charge transfer (CT) between adjacent Fe2+ and Fe3+ cations, while Fe2+→Ti4+ CT has been proposed for blue kyanites (Al2SiO5). Such assignments were based on chemical analyses and on polarization-dependent absorption bands measured in visible-region spectra."[3]
The Fe3+ ions produce golden-yellow color, and when both Fe2+ and Fe3+ are present, the color is a darker blue as in maxixe.[4]
Optical "absorption (OA) spectroscopy [shows] two types of Fe2+."[5]
Micrometeorites

"[T]he carbonaceous material [is] known from observation to dominate the terrestrial [micrometeorite (MM)] flux."[6]
"Ureilites occur about half as often as eucrites (Krot et al. 2003), are relatively friable, have less a wide range of cosmic-ray exposure ages including two less than 1 Myr, and, like the dominant group of MM precursors, contain carbon."[6]
Beta particles
"[M]odels in which γ-rays are absorbed in collisions with X-rays producing nonthermal electron-positron pairs, which in turn radiate further X-rays [have been developed]."[7]
"[T]he reprocessing of radiation by e+ e- pairs could be a sufficiently robust mechanism to yield the canonical spectrum, independent of the details of the particle acceleration mechanism and the parameters of the source, such as the X- and γ-ray luminosity, L, and the size, R."[7]
"[T]he hard X-ray spectrum of a growing number of [active galactic nuclei] AGN [in] the 1-30 keV X-ray emission has four distinct components":[7]
- "an incident power law spectrum with a spectral index αix ≃ 0.9,"[7]
- "an emission line at the energy ~6.4 keV (interpreted as a fluorescent iron K-line),"[7]
- "an absorption edge at 7-8 keV (interpreted as an iron K-edge), and"[7]
- "a broad excess of emission with respect to the underlying power law at energies ≳ 10 keV (interpreted as Compton reflection from cold [T < 106 K, optically thick] material)."[7]
Beta particles (electrons) are more penetrating [than alpha particles], but still can be absorbed by a few millimeters of aluminum. However, in cases where high energy beta particles are emitted shielding must be accomplished with low density materials, e.g. plastic, wood, water or acrylic glass (Plexiglas, Lucite). This is to reduce generation of Bremsstrahlung X-rays. In the case of beta+ radiation (positrons), the gamma radiation from the electron-positron annihilation reaction poses additional concern.
Positrons
In "the spectrum of a middle-aged [pulsar] PSR B0656+14 [may be] two wide, red and blue, flux depressions whose frequency ratio is about 2 and which could be the 1st and 2nd harmonics of electron/positron cyclotron absorption formed at magnetic fields [of] ~108 G in [the] upper magnetosphere of the pulsar."[8]
Electrons
"[W]hen the medium [behaves] like an amplifier to the incident radiation" it is "possible for negative absorption to arise at radio wavelengths".[9]
The necessary and sufficient conditions for negative absorption to occur at radio wavelengths are
- "the kinetic energy distribution F(η) of the radiating electrons [is] markedly non-thermal with an appreciable excess of high energy electrons such that ∂F/∂η is positive over a finite range of the kinetic energy η" and
- "the stimulated transition probability [has] a maximum at some finite value of the kinetic energy, the most favorable case occurring when this maximum is a sharp one at the value of η at which ∂F/∂η has a positive maximum."[9]
"These conditions can both be met in principle for the cases in which the dominant radiation process is due [to]
- [the] Cerenkov effect,
- gyro radiation by non-relativistic electrons, [and]
- synchrotron-type radiation by highly relativistic electrons".[9]
Def. "radiation at the fundamental or at the first few harmonics of the gyro frequency by weakly relativistic electrons rotating in a magnetic field" is called gyro radiation.[9]
Def. "radiation by strongly relativistic electrons at high harmonics of the gyro frequency" is called synchrotron radiation.[9]
Muons
"TeV muons from γ ray primaries ... are rare because they are only produced by higher energy γ rays whose flux is suppressed by the decreasing flux at the source and by absorption on interstellar light."[10]
X-rays
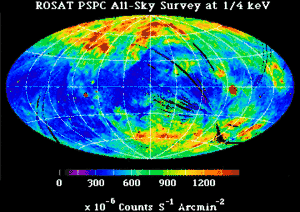
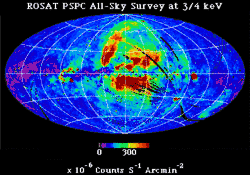
In addition to discrete sources which stand out against the sky, there is good evidence for a diffuse X-ray background.[11] During more than a decade of observations of X-ray emission from the Sun, evidence of the existence of an isotropic X-ray background flux was obtained in 1956.[12] This background flux is rather consistently observed over a wide range of energies.[11] The early high-energy end of the spectrum for this diffuse X-ray background was obtained by instruments on board Ranger 3 and Ranger 5.[11] The X-ray flux corresponds to a total energy density of about 5 x 10−4 eV/cm3.[11] The ROSAT soft X-ray diffuse background (SXRB) image shows the general increase in intensity from the Galactic plane to the poles. At the lowest energies, 0.1 - 0.3 keV, nearly all of the observed soft X-ray background (SXRB) is thermal emission from ~106 K plasma.
By comparing the soft X-ray background with the distribution of neutral hydrogen, it is generally agreed that within the Milky Way disk, super soft X-rays are absorbed by this neutral hydrogen.
There is an “extensive 1/4 keV emission in the Galactic halo”, an “observed 1/4 keV [X-ray emission originating] in a Local Hot Bubble (LHB) that surrounds the Sun. ... and an isotropic extragalactic component.”[13] In addition to this “distribution of emission responsible for the soft X-ray diffuse background (SXRB) ... there are the distinct enhancements of supernova remnants, superbubbles, and clusters of galaxies.”[13]
X-rays in the 0.5 to 5 keV (80 to 800 aJ) range, where most celestial sources give off the bulk of their energy, can be stopped by a few sheets of paper; ninety percent of the photons in a beam of 3 keV (480 aJ) X-rays are absorbed by traveling through just 10 cm of air.
Ultraviolets

Def. "the spectral region bounded on the long wavelength side at about λ3000 by the onset of atmospheric ozone absorption and on the short wavelength side at λ912 by the photoionization of interstellar hydrogen" is called the ultraviolet.[14]
"Vacuum UV" is so named because it is absorbed strongly by air and is, therefore, used in a vacuum. In the long-wave limit of this region, roughly 150–200 nm, the principal absorber is the oxygen in air.
Fluorescences

Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. However, when the absorbed electromagnetic radiation is intense, it is possible for one electron to absorb two photons; this two-photon absorption can lead to emission of radiation having a shorter wavelength than the absorbed radiation. The emitted radiation may also be of the same wavelength as the absorbed radiation, termed "resonance fluorescence".[15]
The most striking examples of fluorescence occur when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, and the emitted light is in the visible region.
The common fluorescent lamp relies on fluorescence. Inside the glass tube is a partial vacuum and a small amount of mercury. An electric discharge in the tube causes the mercury atoms to emit ultraviolet light. The tube is lined with a coating of a fluorescent material, called the phosphor, which absorbs the ultraviolet and re-emits visible light. Fluorescent lighting is more energy-efficient than incandescent lighting elements. However, the uneven spectrum of traditional fluorescent lamps may cause certain colors to appear different than when illuminated by incandescent light or daylight. The mercury vapor emission spectrum is dominated by a short-wave UV line at 254 nm (which provides most of the energy to the phosphors), accompanied by visible light emission at 436 nm (blue), 546 nm (green) and 579 nm (yellow-orange). These three lines can be observed superimposed on the white continuum using a hand spectroscope, for light emitted by the usual white fluorescent tubes. These same visible lines, accompanied by the emission lines of trivalent europium and trivalent terbium, and further accompanied by the emission continuum of divalent europium in the blue region, comprise the more discontinuous light emission of the modern trichromatic phosphor systems used in many compact fluorescent lamp and traditional lamps where better color rendition is a goal.[16]
Ultraviolet lamps are also used in analyzing minerals and gems. Materials may look the same under visible light, but fluoresce to different degrees under ultraviolet light, or may fluoresce differently under short wave ultraviolet versus long wave ultraviolet.
Ultraviolet lamps may cause certain minerals to fluoresce, and is a key tool in prospecting for tungsten mineralisation.
Whites

White is the color of fresh milk and snow.[17][18] It is the color the human eye sees when it looks at light which contains all the wavelengths of the visible spectrum, at full brightness and without absorption. It does not have any hue.[19]
Blacks


Black is the color of coal, ebony, and of outer space. It is the darkest color, the result of the absence of or complete absorption of light. It is the opposite of white and often represents darkness in contrast with light.[20][21][22]
"Opposite to white: colourless from the absence or complete absorption of light. Also, so near this as to have no distinguishable colour, very dark."[20]
Black is "[t]he darkest color".[21]
"Se dit de la couleur la plus foncée, due à l'absence ou à l'absorption totale des rayons lumineux."[22]("said of the very darkest color, due to the absence or complete absorption of all rays of light.")
Yellows
"The GE Reveal bulb is marketed as the bulb that is made to “specially filter out yellow rays that hide life's true colors.” This is accomplished by the use of neodymium in the glass."[23]
Def. "[t]he colour of gold or butter; the colour obtained by mixing green and red light, or by subtracting blue from white light"[24] is called yellow.
Infrareds
| Division Name | Abbreviation | Wavelength | Characteristics |
| Near-infrared | NIR, IR-A DIN | 0.75-1.4 µm | Defined by the water absorption, and commonly used in fiber optic telecommunication because of low attenuation losses in the SiO2 glass (silica) medium. Image intensifiers are sensitive to this area of the spectrum. Examples include night vision devices such as night vision goggles. |
| Short-wavelength infrared | SWIR, IR-B DIN | 1.4-3 µm | Water absorption increases significantly at 1,450 nm. The 1,530 to 1,560 nm range is the dominant spectral region for long-distance telecommunications. |
| Mid-wavelength infrared | MWIR, IR-C DIN. Also called intermediate infrared (IIR) | 3-8 µm | In guided missile technology the 3-5 µm portion of this band is the atmospheric window in which the homing heads of passive IR 'heat seeking' missiles are designed to work, homing on to the Infrared signature of the target aircraft, typically the jet engine exhaust plume |
| Long-wavelength infrared | LWIR, IR-C DIN | 8–15 µm | This is the "thermal imaging" region, in which sensors can obtain a completely passive picture of the outside world based on thermal emissions only and requiring no external light or thermal source such as the sun, moon or infrared illuminator. Forward-looking infrared (FLIR) systems use this area of the spectrum. This region is also called the "thermal infrared." |
| Far infrared | FIR | 15 - 1,000 µm | (see also far-infrared laser). |
Radars
| Band name | Frequency range | Wavelength range | Notes |
|---|---|---|---|
| HF | 3–30 MHz | 10–100 m | Coastal radar systems, over-the-horizon radar (OTH) radars; 'high frequency' |
| VHF | 30–300 MHz | 1–10 m | Very long range, ground penetrating; 'very high frequency' |
| P | < 300 MHz | > 1 m | 'P' for 'previous', applied retrospectively to early radar systems; essentially HF + VHF |
| UHF | 300–1000 MHz | 0.3–1 m | Very long range (e.g. ballistic missile early warning), ground penetrating, foliage penetrating; 'ultra high frequency' |
| L | 1–2 GHz | 15–30 cm | Long range air traffic control and surveillance; 'L' for 'long' |
| S | 2–4 GHz | 7.5–15 cm | Moderate range surveillance, Terminal air traffic control, long-range weather, marine radar; 'S' for 'short' |
| C | 4–8 GHz | 3.75–7.5 cm | Satellite transponders; a compromise (hence 'C') between X and S bands; weather; long range tracking |
| X | 8–12 GHz | 2.5–3.75 cm | Missile guidance, marine radar, weather, medium-resolution mapping and ground surveillance; in the USA the narrow range 10.525 GHz ±25 MHz is used for airport radar; short range tracking. Named X band because the frequency was a secret during WW2. |
| Ku | 12–18 GHz | 1.67–2.5 cm | High-resolution, also used for satellite transponders, frequency under K band (hence 'u') |
| K | 18–24 GHz | 1.11–1.67 cm | From German kurz, meaning 'short'; limited use due to absorption by water vapour, so Ku and Ka were used instead for surveillance. K-band is used for detecting clouds by meteorologists, and by police for detecting speeding motorists. K-band radar guns operate at 24.150 ± 0.100 GHz. |
| Ka | 24–40 GHz | 0.75–1.11 cm | Mapping, short range, airport surveillance; frequency just above K band (hence 'a') Photo radar, used to trigger cameras which take pictures of license plates of cars running red lights, operates at 34.300 ± 0.100 GHz. |
| mm | 40–300 GHz | 1.0–7.5 mm | Millimetre band, subdivided as below. The frequency ranges depend on waveguide size. Multiple letters are assigned to these bands by different groups. These are from Baytron, a now defunct company that made test equipment. |
| V | 40–75 GHz | 4.0–7.5 mm | Very strongly absorbed by atmospheric oxygen, which resonates at 60 GHz. |
| W | 75–110 GHz | 2.7–4.0 mm | Used as a visual sensor for experimental autonomous vehicles, high-resolution meteorological observation, and imaging. |
Gaseous objects
Above the photosphere visible sunlight is free to propagate into space, and its energy escapes the Sun entirely. The change in opacity is due to the decreasing amount of H− ions, which absorb visible light easily.[25] Conversely, the visible light we see is produced as electrons react with hydrogen atoms to produce H− ions.[26][27]
Berylliums
The emission and absorption spectra for beryllium contain lines in the blue.
Borons
The emission and absorption spectra for boron contain lines on the border between violet and blue.
Carbons
Carbon in carbon cluster molecules may have an absorption line at 492 nm. "These are: C; (311 and 348 nm), C-, (447 and 492 nm), and Cy (586 and 643 nm)."[28]
Fluorines
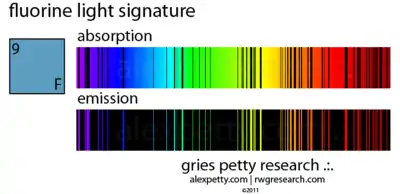
The emission and absorption spectra of fluorine contains at least eight lines or bands from the cyan to the ultraviolet.[29]
Neons
Like fluorine, neon has at least fourteen emission and absorption lines or bands from the cyan to the violet.[30]
Materials
"The depth of the absorption bands and the continuum reflectance of [Kuiper Belt Object] 1996 TO66 suggest the presence of a black- to slightly blue-colored, spectrally featureless particulate material as a minority component mixed with the water ice."[31]
Earth
"[P]referential absorption of sunlight by ozone over long horizon paths gives the zenith sky its blueness when the sun is near the horizon".[32]
Mars
"Several types of rock surface materials can be recognized at the two sites [Viking Lander 1 and Viking Lander 2]; dark, relatively 'blue' rock surfaces are probably minimally weathered igneous rock, whereas bright rock surfaces, with a green/(blue + red) ratio higher than that of any other surface material, are interpreted as a weathering product formed in situ on the rock."[33]
Asteroids


At top right is an approximately natural color image of the asteroid 243 Ida.
"There are brighter areas, appearing bluish in the picture, around craters on the upper left end of Ida, around the small bright crater near the center of the asteroid, and near the upper right-hand edge (the limb). This is a combination of more reflected blue light and greater absorption of near infrared light, suggesting a difference in the abundance or composition of iron-bearing minerals in these areas."[34]
"The [Sloan Digital Sky Survey] SDSS “blue” asteroids are related to the C-type (carbonaceous) asteroids, but not all of them are C-type. They are a mixture of C-, E-, M-, and P-types."[35]
Pallas, minor-planet designation 2 Pallas, is the second asteroid to have been discovered (after Ceres), and one of the largest in the Solar System. It is estimated to comprise 7% of the mass of the asteroid belt,[36] and its diameter of 544 kilometres (338 mi) is slightly larger than that of 4 Vesta. It is however 10–30% less massive than Vesta,[37] placing it third among the asteroids.
"Spectrally blue (B-type) asteroids are rare, with the second discovered asteroid, Pallas, being the largest and most famous example."[38]
"[T]he negative optical spectral slope of some B-type asteroids is due to the presence of a broad absorption band centered near 1.0 μm. The 1 μm band can be matched in position and shape using magnetite (Fe3O4), which is an important indicator of past aqueous alteration in the parent body. ... Observations of B-type asteroid (335) Roberta in the 3 μm region reveal an absorption feature centered at 2.9 μm, which is consistent with the absorption due to phyllosilicates (another hydration product) observed in CI chondrites. ... at least some B-type asteroids are likely to have incorporated significant amounts of water ice and to have experienced intensive aqueous alteration."[38]
G-type asteroids are a relatively uncommon type of carbonaceous asteroid. The most notable asteroid in this class is 1 Ceres. Generally similar to the C-type objects, but containing a strong ultraviolet absorption feature below 0.5 μm.
Callisto
Callisto has a very tenuous atmosphere composed of carbon dioxide.[39] It was detected by the Galileo Near Infrared Mapping Spectrometer (NIMS) from its absorption feature near the wavelength 4.2 micrometers. The surface pressure is estimated to be 7.5 x 10-12 bar (0.75 µPa) and particle density 4 x 108 cm−3. Because such a thin atmosphere would be lost in only about 4 days (see atmospheric escape), it must be constantly replenished, possibly by slow sublimation of carbon dioxide ice from the satellite's icy crust,[39] which would be compatible with the sublimation–degradation hypothesis for the formation of the surface knobs.
Uranus

The middle layer of the Uranian atmosphere is the stratosphere, where temperature generally increases with altitude from 53 K in the tropopause to between 800 and 850 K at the base of the thermosphere.[40] The heating of the stratosphere is caused by absorption of solar UV and IR radiation by methane and other hydrocarbons,[41] which form in this part of the atmosphere as a result of methane photolysis.[42] Heat is also conducted from the hot thermosphere.[41] The hydrocarbons occupy a relatively narrow layer at altitudes of between 100 and 300 km corresponding to a pressure range of 10 to 0.1 mbar (1000 to 10 kPa) and temperatures of between 75 and 170 K.[43][44] The most abundant hydrocarbons are methane, acetylene and ethane with mixing ratios of around 10−7 relative to hydrogen. The mixing ratio of carbon monoxide is similar at these altitudes.[43][44][45] Heavier hydrocarbons and carbon dioxide have mixing ratios three orders of magnitude lower.[44] The abundance ratio of water is around 7×10−9.[46] Ethane and acetylene tend to condense in the colder lower part of stratosphere and tropopause (below 10 mBar level) forming haze layers,[42] which may be partly responsible for the bland appearance of Uranus. The concentration of hydrocarbons in the Uranian stratosphere above the haze is significantly lower than in the stratospheres of the other giant planets.[43][47]
Neptune

At high altitudes, Neptune's atmosphere is 80% hydrogen and 19% helium.[48] A trace amount of methane is also present. Prominent absorption bands of methane occur at wavelengths above 600 nm, in the red and infrared portion of the spectrum. As with Uranus, this absorption of red light by the atmospheric methane is part of what gives Neptune its blue hue,[49] although Neptune's vivid azure differs from Uranus's milder cyan. Since Neptune's atmospheric methane content is similar to that of Uranus, some unknown atmospheric constituent is thought to contribute to Neptune's colour.[50]
Dark nebulae
"The 111 → 110 rotational transition of formaldehyde (H2CO) [occurs] in absorption in the direction of four dark nebulae. The radiation ... being absorbed appears to be the isotropic microwave background".[51] One of the dark nebulae sampled, per SIMBAD is TGU H1211 P5.
Technology
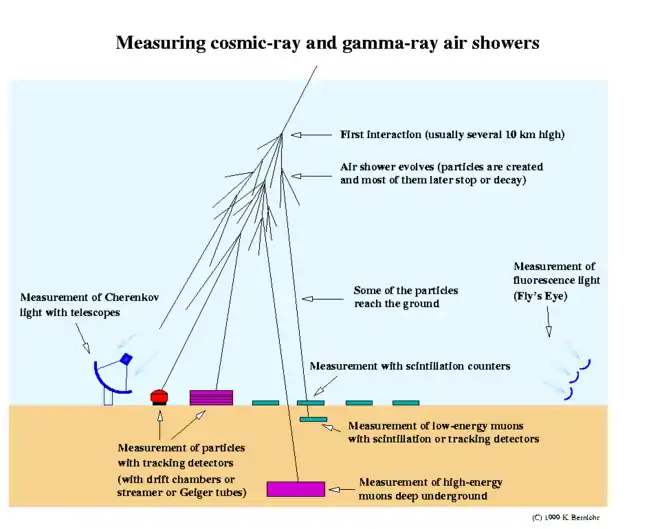
"The Cherenkov telescopes do not actually detect the gamma rays directly but instead detect the flashes of visible light produced when gamma rays are absorbed by the Earth's atmosphere.[52]
Galileo Orbiter

The Energetic Particles Detector (EPD) aboard the Galileo Orbiter is designed to measure the numbers and energies of electrons whose energies exceed about 20 keV. The EPD [can] also measure the direction of travel of [electrons]. The EPD [uses] silicon solid state detectors and a time-of-flight detector system to measure changes in the energetic [electron] population at Jupiter as a function of position and time.
"[The] two bi-directional, solid-state detector telescopes [are] mounted on a platform which [is] rotated by a stepper motor into one of eight positions. This rotation of the platform, combined with the spinning of the orbiter in a plane perpendicular to the platform rotation, [permits] a 4-pi [or 4π] steradian coverage of incoming [electrons]. The forward (0 degree) ends of the two telescopes [have] an unobstructed view over the [4π] sphere or [can] be positioned behind a shield which not only [prevents] the entrance of incoming radiation, but [contains] a source, thus allowing background corrections and in-flight calibrations to be made. ... The 0 degree end of the [Low-Energy Magnetospheric Measurements System] LEMMS [uses] magnetic deflection to separate incoming electrons and ions. The 180 degree end [uses] absorbers in combination with the detectors to provide measurements of higher-energy electrons ... The LEMMS [provides] measurements of electrons from 15 keV to greater than 11 MeV ... in 32 rate channels."[53]
Imaging Compton Telescope

"COMPTEL's upper layer of detectors are filled with a liquid scintillator which scatters an incoming gamma-ray photon according to the Compton Effect. This photon is then absorbed by NaI crystals in the lower detectors. The instrument records the time, location, and energy of the events in each layer of detectors which makes it possible to determine the direction and energy of the original gamma-ray photon and reconstruct an image and energy spectrum of the source."[54]
Scintillation detectors
A scintillator is a material, which exhibits scintillation—the property of luminescence[55] when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate, i.e., reemit the absorbed energy in the form of light. Here, "particle" refers to "ionizing radiation" and can refer either to charged particulate radiation, such as electrons and heavy charged particles, or to uncharged radiation, such as photons and neutrons, provided that they have enough energy to induce ionization.
A scintillation detector or scintillation counter is obtained when a scintillator is coupled to an electronic light sensor such as a photomultiplier tube (PMT) or a photodiode. PMTs absorb the light emitted by the scintillator and reemit it in the form of electrons via the photoelectric effect. The subsequent multiplication of those electrons (sometimes called photo-electrons) results in an electrical pulse which can then be analyzed and yield meaningful information about the particle that originally struck the scintillator.
See also
- Background astronomy
- Portal:Radiation astronomy
- Band astronomy
- Emission astronomy
- Intensity astronomy
- Lensing astronomy
- Reflection astronomy
- Refraction astronomy
- Scattering astronomy
- Spallation astronomy
- Transduction astronomy
- Transmutation astronomy
References
- ↑ Harvey, Eveline (14 January 2011). "NZ blue ice sighting an unexpected treat for tourists". The New Zealand Herald. http://www.nzherald.co.nz/travel/news/article.cfm?c_id=7&objectid=10699700. Retrieved 21 September 2011.
- ↑ Why Is Water Blue
- ↑ Kathleen M. Parkin; Bruce M. Loeffler; Roger G. Burns (09 1977). "Mössbauer spectra of kyanite, aquamarine, and cordierite showing intervalence charge transfer". Physics and Chemistry of Minerals 1 (3): 301-311. doi:10.1007/BF00307569. http://adsabs.harvard.edu/abs/1977PCM.....1..301P. Retrieved 2017-01-21.
- ↑ Color in the Beryl group. Retrieved 2009-06-06.
- ↑ Ana Regina Blak; Sadao Isotani; Shigueo Watanabe (1983). "Optical absorption and electron spin resonance in blue and green natural beryl: A reply". Physics and Chemistry of Minerals 9 (6): 279. doi:10.1007/BF00309581.
- 1 2 Susan Taylor; Gregory F. Herzog; Jeremy S. Delaney (2007). "Crumbs from the crust of Vesta: Achondritic cosmic spherules from the South Pole water well". Meteoritics & Planetary Science 42 (2): 223-33. doi:10.1111/j.1945-5100.2007.tb00229.x.
- 1 2 3 4 5 6 7 Andrzej A. Zdziarski; Gabriele Ghisellini; Ian M. George; R. Svensson; A. C. Fabian; Chris Done (November 1, 1990). "Electron-positron pairs, Compton reflection, and the X-ray spectra of active galactic nuclei". The Astrophysical Journal 363 (11): L1-4. doi:10.1086/185851. http://adsabs.harvard.edu/full/1990ApJ...363L...1Z. Retrieved 2013-08-15.
- ↑ S. Zharikov; R. E. Mennickent; Yu. Shibanov; V. Komarova (April 2007). "Optical spectroscopy of the radio pulsar PSR B0656+14". Astrophysics and Space Science 308 (1-4): 545-9. doi:10.1007/s10509-007-9308-z. http://adsabs.harvard.edu/abs/2007Ap%26SS.308..545Z. Retrieved 2013-05-31.
- 1 2 3 4 5 R. Q. Twiss (December 1958). "Radiation Transfer and the Possibility of Negative Absorption in Radio Astronomy". Australian Journal of Physics 11 (12): 564.
- ↑ Francis Halzen; Todor Stanev; Gaurang B. Yodh (April 1, 1997). "γ ray astronomy with muons". Physical Review D Particles, Fields, Gravitation, and Cosmology 55 (7): 4475-9. doi:10.1103/PhysRevD.55.4475. http://prd.aps.org/abstract/PRD/v55/i7/p4475_1. Retrieved 2013-01-18.
- 1 2 3 4 Morrison P (1967). "Extrasolar X-ray Sources". Ann Rev Astron Astrophys. 5 (1): 325–50. doi:10.1146/annurev.aa.05.090167.001545.
- ↑ Kupperian JE Jr, Friedman H (1958). "Experiment research US progr. for IGY to 1.7.58". IGY Rocket Report Ser. (1): 201.
- 1 2 S. L. Snowden; R. Egger; D. P. Finkbiner; M. J. Freyberg; P. P. Plucinsky (February 1, 1998). "Progress on Establishing the Spatial Distribution of Material Responsible for the 1/4 keV Soft X-Ray Diffuse Background Local and Halo Components". The Astrophysical Journal 493 (1): 715-29. doi:10.1086/305135. http://iopscience.iop.org/0004-637X/493/2/715/fulltext/. Retrieved 2012-06-14.
- ↑ R. C. Bless; A. D. Code (1972). "Ultraviolet Astronomy". Annual Review of Astronomy and Astrophysics 10: 197-226. doi:10.1146/annurev.aa.10.090172.001213.
- ↑ Principles Of Instrumental Analysis F.James Holler, Douglas A. Skoog & Stanley R. Crouch 2006
- ↑ Tom Harris. How Fluorescent Lamps Work, In: How Stuff Works. Discovery Communications. Retrieved 27 June 2010.
- ↑ Shorter Oxford English Dictionary, 5th Edition (2002)
- ↑ See Shorter Oxford English Dictionary, 5th Edition (2002); "of the colour of fresh milk or snow." See also Webster's New World Dictionary of American English, Third College Edition, (1988): "Having the color of pure snow or milk." See also The Random House College Dictionary of the English Language, Revised Edition,(1980)
- ↑ Shorter Oxford English Dictionary, 5th Edition (2002)
- 1 2 Shorter Oxford English Dictionary. 5th Edition (2002)
- 1 2 See also Webster's New World Dictionary of the American Language (1964)
- 1 2 Le Petit Larousse Illustré (1997)
- ↑ Jennifer J. Birriel (September 2008). "Demonstrating Absorption Spectra Using Commercially Available Incandescent Light Bulbs". Astronomy Education Review 7 (2): 147-57. doi:10.3847/AER2008035. http://link.aip.org/link/?AERSCZ/7/147/1. Retrieved 2013-09-13.
- ↑ yellow. San Francisco, California: Wikimedia Foundation, Inc. September 1, 2013. Retrieved 2013-09-02.
- ↑ K.D. Abhyankar (1977). "A Survey of the Solar Atmospheric Models". Bull. Astr. Soc. India 5: 40–44. http://prints.iiap.res.in/handle/2248/510.
- ↑ E.G. Gibson (1973). The Quiet Sun. NASA. ASIN B0006C7RS0.
- ↑ Shu, F.H. (1991). The Physics of Astrophysics. 1. University Science Books. ISBN 0-935702-64-4.
- ↑ W Krätschmer; N Sorg; Donald R. Huffman (June 3, 1985). "Spectroscopy of matrix-isolated carbon cluster molecules between 200 and 850 nm wavelength". Surface Science 156 (2): 814-21. doi:10.1016/0039-6028(85)90253-5. http://www.sciencedirect.com/science/article/pii/0039602885902535. Retrieved 2012-07-30.
- ↑ Alex Petty (July 2007). Fluorine light signature. alexpetty.com. Retrieved 2013-06-01.
- ↑ Alex Petty (July 2011). Neon light signature. alexpetty.com. Retrieved 2013-06-01.
- ↑ Robert H. Brown; Dale P. Cruikshank; Yvonne Pendleton (July 1, 1999). "Water Ice on Kuiper Belt Object 1996 TO66". The Astrophysical Journal 519 (1): L101-4. doi:10.1086/312098. http://iopscience.iop.org/1538-4357/519/1/L101/fulltext/. Retrieved 2013-06-01.
- ↑ Craig F. Bohren. Atmospheric Optics (PDF).
- ↑ Edwin L. Strickland III (March 19-23 1979). Martian soil stratigraphy and rock coatings observed in color-enhanced Viking Lander images, In: Lunar and Planetary Science Conference Proceedings. 3. New York: Pergamon Press, Inc.. pp. 3055-77. http://adsabs.harvard.edu/abs/1979LPSC...10.3055S. Retrieved 2013-05-31.
- ↑ Sue Lavoie (January 29, 1996). PIA00069: Ida and Dactyl in Enhanced Color. Pasadena, California USA: NASA/JPL. Retrieved 2013-06-01.
- ↑ F Yoshida, T Nakamura (June 2007). "Subaru main belt asteroid survey (SMBAS)—size and color distributions of small main-belt asteroids". Planetary and Space Science 55 (9): 1113-25. doi:10.1016/j.pss.2006.11.016. http://www.sciencedirect.com/science/article/pii/S0032063306003357. Retrieved 2013-06-01.
- ↑ Elena V. Pitjeva (2005). "High-Precision Ephemerides of Planets—EPM and Determination of Some Astronomical Constants". Solar System Research 39 (3): 176. doi:10.1007/s11208-005-0033-2. http://iau-comm4.jpl.nasa.gov/EPM2004.pdf.
- ↑ James Baer; Steven R. Chesley (2008). "Astrometric masses of 21 asteroids, and an integrated asteroid ephemeris". Celestial Mechanics and Dynamical Astronomy 100 (2008): 27–42. doi:10.1007/s10569-007-9103-8. http://www.springerlink.com/content/h747307j43863228/fulltext.pdf. Retrieved 2008-11-11.
- 1 2 Bin Yang; David Jewitt (September 2010). "Identification of Magnetite in B-type Asteroids". The Astronomical Journal 140 (3): 692. doi:10.1088/0004-6256/140/3/692. http://iopscience.iop.org/1538-3881/140/3/692. Retrieved 2013-06-01.
- 1 2 Carlson, R. W.; et al. (1999). "A Tenuous Carbon Dioxide Atmosphere on Jupiter's Moon Callisto" (PDF). Science 283 (5403): 820–821. doi:10.1126/science.283.5403.820. PMID 9933159. http://trs-new.jpl.nasa.gov/dspace/bitstream/2014/16785/1/99-0186.pdf.
- ↑
doi: 10.1029/JA092iA13p15093
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - 1 2 Young, Leslie A.; Bosh, Amanda S.; Buie, Marc; Elliot, J. L.; Wasserman, Lawrence H. (2001). et al. 2001Uranus.pdf "Uranus after Solstice: Results from the 1998 November 6 Occultation" (PDF). Icarus 153 (2): 236–247. doi:10.1006/icar.2001.6698. http://www.boulder.swri.edu/~layoung/eprint/ur149/Young et al. 2001Uranus.pdf.
- 1 2
doi: 10.1086/168031
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - 1 2 3
doi: 10.1016/0019-1035(90)90094-P
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - 1 2 3
doi: 10.1016/j.icarus.2006.06.006
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑
doi: 10.1051/0004-6361:20034637
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑
doi: 10.1016/S0032-0633(02)00145-9
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑
doi: 10.1016/S0032-0633(98)00142-1
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Hubbard, W. B. (1997). "Neptune's Deep Chemistry". Science 275 (5304): 1279–1280. doi:10.1126/science.275.5304.1279. PMID 9064785.
- ↑ D. Crisp; H. B. Hammel (14 June 1995). Hubble Space Telescope Observations of Neptune. Hubble News Center. Retrieved 22 April 2007.
- ↑ Kirk Munsell; Smith, Harman; Harvey, Samantha (13 November 2007). Neptune overview, In: Solar System Exploration. NASA. Retrieved 20 February 2008.
- ↑ Patrick Palmer; B. Zuckerman; David Buhl; Lewis E. Snyder (June 1969). "Formaldehyde Absorption in Dark Nebulae". The Astrophysical Journal 156 (6): L147-50. doi:10.1086/180368.
- ↑ Margaret J. Penston (14 August 2002). The electromagnetic spectrum. Particle Physics and Astronomy Research Council. Retrieved 17 August 2006.
- ↑ Donald J. Williams (May 14, 2012). Energetic Particles Detector (EPD). Greenbelt, Maryland USA: NASA Goddard Space Flight Center. Retrieved 2012-08-11.
- ↑ Neil Gehrels (August 1, 2005). The Imaging Compton Telescope (COMPTEL). Greenbelt, Maryland USA: NASA Goddard Space Flight Center. Retrieved 2013-04-05.
- ↑ Leo, W. R. (1994). “Techniques for Nuclear and particle Physics Experiments”, 2nd edition, Springer, ISBN 978-3540572800
