The term dominant group appears to serve as an identifier used by authors of chemistry refereed journal articles to indicate an observation of phenomena in the process of analysis. At the more general level it may be a chemical entity such as a chemist, laboratory, or chemical species, or associated with chemistry in some way.
Chemistry
Def. a science that deals with
- the identification of the substances of which matter is composed,
- the investigation of their properties and the ways in which they interact, combine, and change, and
- the use of these processes to form new substances
is called chemistry.
Def. "a science that deals with the composition, structure, and properties of substances and of the transformations that they undergo"[1] is called chemistry.
"Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties.[2][3] Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds."[4]
Dominant groups
- Accident hypothesis: dominant group is an accident of whatever processes are operating.
- Artifact hypothesis: dominant group may be an artifact of human endeavor or may have preceded humanity.
- Association hypothesis: dominant group is associated in some way with the original research.
- Bad group hypothesis: dominant group is the group that engages in discrimination, abuse, punishment, and additional criminal activity against other groups. It often has an unfair advantage and uses it to express monopolistic practices.
- Control group hypothesis: there is a control group that can be used to study dominant group.
- Entity hypothesis: dominant group is an entity within each field where a primary author of original research uses the term.
- Evolution hypothesis: dominant group is a product of evolutionary processes, such groups are the evolutionary process, produce evolutionary processes, or are independent of evolutionary processes.
- Identifier hypothesis: dominant group is an identifier used by primary source authors of original research to identify an observation in the process of analysis.
- Importance hypothesis: dominant group signifies original research results that usually need to be explained by theory and interpretation of experiments.
- Indicator hypothesis: dominant group may be an indicator of something as yet not understood by the primary author of original research.
- Influence hypothesis: dominant group is included in a primary source article containing original research to indicate influence or an influential phenomenon.
- Interest hypothesis: dominant group is a theoretical entity used by scholarly authors of primary sources for phenomena of interest.
- Metadefinition hypothesis: all uses of dominant group by all primary source authors of original research are included in the metadefinition for dominant group.
- Null hypothesis: there is no significant or special meaning of dominant group in any sentence or figure caption in any refereed journal article.
- Object hypothesis: dominant group is an object within each field where a primary author of original research uses the term.
- Obvious hypothesis: the only meaning of dominant group is the one found in Mosby's Medical Dictionary.
- Original research hypothesis: dominant group is included in a primary source article by the author to indicate that the article contains original research.
- Primordial hypothesis: dominant group is a primordial concept inherent to humans such that every language or other form of communication no matter how old or whether extinct, on the verge of extinction, or not, has at least a synonym for dominant group.
- Purpose hypothesis: dominant group is written into articles by authors for a purpose.
- Regional hypothesis: dominant group, when it occurs, is only a manifestation of the limitations within a region. Variation of those limitations may result in the loss of a dominant group with the eventual appearance of a new one or none at all.
- Source hypothesis: dominant group is a source within each field where a primary author of original research uses the term.
- Term hypothesis: dominant group is a significant term that may require a 'rigorous definition' or application and verification of an empirical definition.
Examples from primary sources are to be used to prove or disprove each hypothesis. These can be collected per subject or in general.
Sciences
Def. the disciplines or branches of learning, especially those "dealing with measurable or systematic principles ... the collective [disciplines] of study or learning acquired through the scientific method"[5] are called the sciences.
Theory chemistry
Def. a "branch of natural science that deals with the composition and constitution of substances and the changes that they undergo as a consequence of alterations in the constitution of their molecules"[6] is called chemistry.
Entities
"A major problem for the increasingly dominant group of university teachers was how to combine — or even merge — their professional duties with their self-image as scientists."[7]
Chemicals
"At higher growth temperatures(>600'C) the lifetimes of the alkyl-gallium species are much shorter and the growth front dynamics should begin to look more like MBE since atomic gallium will be the dominant group III surface species."[8]
"The blue shift effect which is often observed in multiple quantum well (MQW) structures subjected to heat treatment, is attributed to a dominant group V interdiffusion which can be suppressed by high defect densities in the substrate."[9]
Hydrogens
"Use of an appropriate hydrogen level is necessary to favor dehalogenation of chlorinated solvents, such as tetrachloroethene (PCE) and trichloroethene (TCE), over other hydrogen using processes. ... the competition between dehalogenators and other microorganisms occurring in a benzoate-acclimated dehalogenating methanogenic mixed culture. ... the dehalogenators competed best against methanogens and homoacetogens when the hydrogen level was maintained between 2 and 11 nM."[10]
"In contrast, with hydrogen as the primary electron donor, homoacetogens became the dominant group in hydrogen utilization with their advantageous kinetic properties."[10]
Heliums
"Helium, U, V, and Se make up the dominant group, giving rise to highly anomalous He and U and weakly anomalous V and Se patterns."[11]
Lithiums
"In the other series of glasses, Li, Na, and K, in particular, that can be formed out to x=0.75, the IR spectra clearly show that this structural group becomes the dominant group in the glass at this composition [4], [5] and [43]."[12]
Berylliums
Notation: let the symbol DPM represent Diesel vehicle exhaust Particulate Matter.
"Moreover DPM can bear metals like antimony, arsenic, beryllium, cadmium, chromium, copper, iron, lead, magnesium, manganese, nickel, vanadium and zinc ... the dominant group of particles had a diameter of 20 to 25 nm (at 900 rpm 50% load or 25 to 30 nm 100% load) for individual soot nodules."[13]
Borons
For the "reedmergnerite unit, BSi4O10 ... The spectra showed small amounts of it, and we saw no evidence of fragmentation (i.e., no corresponding smaller fragments that would appear to be constituents). Thus, it does not appear to be the dominant group in the glass network. ... The borate association remains dominant, however, even as we increase the soda content."[14]
Carbons
"In general, during 1990-1991 most of the carbon biomass was contributed by diatoms, while during 1992-1993 it was contributed mainly by flagellates, although during Leg II of 1993 dinoflagellates were the dominant group in terms of carbon content."[15]
Arsenics
"The dominant group V source is arsenic, although antimony and phosphorous sources are not atypical."[16]
Compounds
Notation: let the symbol PE stand for polyethylene.
"Finally, there is a dominant group (PE) that is still growing on the market."[17]
"Among a variety of tool making materials, sintered carbides are still a dominant group in view of machining technologies. ... Having considered all types of coating materials, the most numerous group is made up by materials with the predominant number of metallic bonds."[18]
Ions
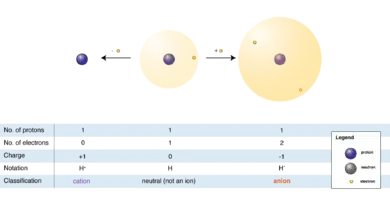
"An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge."[19]
The hydrogen anion (shown in the diagram at right), with its loosely held two-electron cloud, has a larger radius than the neutral atom, which in turn is much larger than the bare proton of the cation. Hydrogen forms the only cation that has no electrons, but even cations that (unlike hydrogen) retain one or more electrons, are smaller than the neutral atoms or molecules from which they are derived.
"In the second dominant group of ions we generally see more discrepancies in the model and the HIS data".[20]
Molecules
"The ω-hydroxymonocarboxylates represented the dominant group with a contribution higher than one quarter of the total."[21]
Agrochemistry
"Agricultural chemistry is the study of both chemistry and biochemistry which are important in agricultural production, the processing of raw products into foods and beverages, and in environmental monitoring and remediation. These studies emphasize the relationships between plants, animals and bacteria and their environment."[22]
"Agricultural chemistry often aims at preserving or increasing the fertility of soil, maintaining or improving the agricultural yield, and improving the quality of the crop."[22]
"In this substitution process heterocyclic products became the dominant group of development candidates for protecting crops against pathogens, insects, mites and weed competition in the 1990ies(6)."[23]
Notation: let the symbol HA stand for humic acid.
"HAs have been the main object of study in the investigated soils primarily because, from an ecological point of view, this is the most stable, though not always dominant group of substances."[24]
"Phosphomonoesters are the dominant group of organic phosphorus compounds in most soils and comprise 16%–55% of total soil P."[25]
Analytical chemistry
"Analytical chemistry is the analysis of material samples to gain an understanding of their chemical composition and structure. Analytical chemistry incorporates standardized experimental methods in chemistry. These methods may be used in all subdisciplines of chemistry, excluding purely theoretical chemistry."[4]
"Br and Pb were more evenly distributed over the various food groups; the group 'nuts' showed the highest content of Br (about 8 mg/kg), but for Pb such a dominant group could not be indicated."[26]
"Various oxygen functionalities include carboxyl (−COOH), the dominant group, and phenolic and/or enolic −OH, alcoholic −OH, and carbonyl (−CO)."[27]
"In sediments, total PCT concentrations expressed as A5442 equivalents ranged from 22 to 100 μg/kg of wet weight, with hepta-CTs as the dominant group of congeners."[28]
"According to their composition, the Japanese cultivars can be categorized into two groups on the basis of the shade of color and the peonidin/cyanidin (pn/cy) ratio: cyanidin types (pn/cy < 1.0) with a greater degree of blueness (blue dominant group) and peonidin types (pn/cy > 1.0) with a greater degree of redness (red dominant group) (9, 16)."[29]
"On the basis of the calculated peonidin/cyanidin ratio (0.10) this cultivar belongs to the blue dominant group (“cyanidin-type”)."[29]
Biochemistry
"Biochemistry is the study of the chemicals, chemical reactions and chemical interactions that take place in living organisms. Biochemistry and organic chemistry are closely related, as in medicinal chemistry or neurochemistry. Biochemistry is also associated with molecular biology and genetics."[4]
Notation: let the symbol EBPR stand for the enhanced biological phosphate removal process.
"Conventionally, it has been assumed that EBPR sludges with high P removal capability would be enriched with a single dominant group of microorganisms."[30]
"However, these monosaccharides failed to inhibit significantly the quantitative precipitin reactions between purified antigen and type Ia antiserum. Indications are that the immunodominant group of this antigen consists of more than a simple monosaccharide."[31]
"The second dominant group in the A sludge was the Actinobacteria."[32]
Inorganic chemistry
"Inorganic chemistry is the study of the properties and reactions of inorganic compounds. The distinction between organic and inorganic disciplines is not absolute and there is much overlap, most importantly in the sub-discipline of organometallic chemistry."[4]
"The behavior of both moieties could be successfully described by the L component of the TLS analysis, with the T and S components having negligible magnitudes, indicating that libration about the central atom was the dominant group motion for both species."[33]
"The sterically dominant group in the O,NTf chiral ligand is the SO 2 moiety, as one oxygen protrudes into the open quadrant created by one of the back pointing pseudoaxial P-phenyl substituents."[34]
"Both of the emission spectra present the characteristic emission bands originating from the transition 5D4 → 7FJ (J=6, 5, 4, 3), with the transition 5D4 → 7F5 green emission as the dominant group.[35]
Neurochemistry
"Neurochemistry is the study of neurochemicals; including transmitters, peptides, proteins, lipids, sugars, and nucleic acids; their interactions, and the roles they play in forming, maintaining, and modifying the nervous system."[4]
"Plasma levels of activity in the autosomal dominant group (1700 ± 90 U) were higher than those in the control (850 ± 60 U) or the autosomal recessive group (660 ± 70 U) (p less than 0.001)."[36]
Nuclear chemistry
"Nuclear chemistry is the study of how subatomic particles come together and make nuclei. Modern Transmutation is a large component of nuclear chemistry, and the table of nuclides is an important result and tool for this field."[4]
"The most dominant group of particles mostly consisted of Potassium and Potassium/Potassium Sulfate particle types, comprising 54% of the collected spectra during the 10 days."[37]
"Since the energy of the 3.11 MeV peak observed here coincides with that of the dominant group in the delayed proton spectrum of Ca, it has been necessary to evaluate the possibility that Ca, produced in reactions on the small oxygen contamination in the target, could be the source of this activity"[38]
Organic chemistry

"Organic chemistry is the study of the structure, properties, composition, mechanisms, and reactions of organic compounds. An organic compound is defined as any compound based on a carbon skeleton."[4]
"Overall, polyunsaturated fatty acids were the dominant group of fatty acids (Table 2), except at Station 6 where saturated fatty acids also dominated the fatty acid pool."[39]
"The four model compounds selected represent pyridinic and CN nitrogen groups, pyridinic nitrogen because this group has been observed to be a dominant group and CN because of the indirect observation of HCN formation."[40]
"In contrast, with hydrogen as the primary electron donor, homoacetogens became the dominant group in hydrogen utilization with their advantageous kinetic properties."[10]
"[T]he dominant group is the same in each case and the subsidiary group is changed only by the substitution of acetyl for methyl at the oxygen atom."[41]
"It appears to us that the only interpretation that can be placed upon the facts as they are now known to us is that the acceptor is a radicle which is very closely allied to, if not identical with, a dominant group in the hydrolyte."[42]
"In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of.[43] However, its relative reactivity can be modified by nearby functional groups."[44] "In diffuse clouds the dominant group appears to be -CH3."[45]
"An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH."[46]
"That good correlation can be obtained if one focuses attention on the dominant group can be nicely illustrated with the benzoic acids."[47]
"Volatile organic compounds (VOCs) are organic chemicals that have a high vapor pressure at ordinary, room-temperature conditions. Their high vapor pressure results from a low boiling point, which causes large numbers of molecules to evaporate or sublimate from the liquid or solid form of the compound and enter the surrounding air."[48]
"As such alcohols became the dominant group of volatiles in the overripe mango, the relative concentrations of other groups of compounds were, of course, correspondingly reduced."[49]
Physical chemistry
"Physical chemistry is the study of the physical and fundamental basis of chemical systems and processes. In particular, the energetics and dynamics of such systems and processes are of interest to physical chemists. Important areas of study include chemical thermodynamics, chemical kinetics, electrochemistry, statistical mechanics, spectroscopy, and more recently, astrochemistry.[50] Physical chemistry has large overlap with molecular physics. Physical chemistry involves the use of infinitesimal calculus in deriving equations. It is usually associated with quantum chemistry and theoretical chemistry. Physical chemistry is a distinct discipline from chemical physics, but again, there is very strong overlap."[4]
"The P species in the titania lattice should be the dominant group responsive to visible light, whereas the P species on the surface could retard the phase transition of anatase to rutile and increase the surface area and adsorption capability."[51]
"The P species in the titania lattice should be the dominant group responsive to the visible light activity, although P species on the surface could retard the phase transition of anatase to rutile, and increase the surface area and adsorption capability, consequently enhancing the photocatalytic activity."[51]
Theoretical chemistry
"Theoretical chemistry is the study of chemistry via fundamental theoretical reasoning (usually within mathematics or physics). In particular the application of quantum mechanics to chemistry is called quantum chemistry. Since the end of the Second World War, the development of computers has allowed a systematic development of computational chemistry, which is the art of developing and applying computer programs for solving chemical problems. Theoretical chemistry has large overlap with (theoretical and experimental) condensed matter physics and molecular physics."[4]
"Molecular biologists, as the dominant group after the Second World War, made great advances (aided by ample financial support) and sometimes became quite arrogant."[52]
Hypotheses
- A dominant group in chemistry is as likely to be a group of chemists as it is a group of molecules.
See also
References
- ↑ Philip B. Gove, ed. (1963). Webster's Seventh New Collegiate Dictionary. Springfield, Massachusetts: G. & C. Merriam Company. p. 1221.
- ↑ "What is Chemistry?". Chemweb.ucc.ie. Retrieved 2011-06-12.
- ↑ Chemistry. (n.d.). Merriam-Webster's Medical Dictionary. Retrieved August 19, 2007.
- 1 2 3 4 5 6 7 8 9 "Chemistry, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 27, 2012. Retrieved 2012-07-15.
- ↑ "science, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. February 15, 2013. Retrieved 2013-02-19.
- ↑ Paul G (23 February 2004). "chemistry, In: Wiktionary". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2016-08-25.
- ↑ Jan Frercks (March 2010). "Demonstrating the Facticity of Facts: University Lectures and Chemistry as a Science in Germany around 1800". Ambix 57 (1): 64-83. doi:10.1179/174582310X12629173850005. http://www.ingentaconnect.com/content/maney/amb/2010/00000057/00000001/art00005. Retrieved 2012-07-17.
- ↑ A. Robertson Jr, T.H. Chiu, W.T. Tsang and J.E. Cunningham (1987). "RHEED Intensity Oscillation Studies of the Kinetics of GaAs Deposition During Chemical Beam Epitaxy (CBE)". MRS Proceedings 102: 17-23. doi:http://dx.doi.org/10.1557/PROC-102-17. http://journals.cambridge.org/abstract_S1946427400538469. Retrieved 2012-07-17.
- ↑ S. L. Wong, R. J. Nicholas, R. W. Martin, J. Thompson, A. Wood, A. Moseley, N. Carr (May 1996). "A magneto‐optical study of interdiffusion in InGaAs/InP quantum wells: Effects of heat treatment, substrates, and dopants". Journal of Applied Physics 79 (9): 6826-33. http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=5015589. Retrieved 2012-07-17.
- 1 2 3 Yanru Yang and Perry L. McCarty (September 26, 1998). "Competition for Hydrogen within a Chlorinated Solvent Dehalogenating Anaerobic Mixed Culture". Environmental Science & Technology 32 (22): 3591-7. doi:10.1021/es980363n. http://lib3.dss.go.th/fulltext/Journal/Environ%20Sci.%20Technology1998-2001/1998/no.22/22,1998%20vol.32,no.22,p3591-3597.pdf. Retrieved 2012-03-16.
- ↑ Willy Dyck, Dwaine Car (June 1987). "Detailed geochemical studies of a He-U lake anomaly in permafrost, Baker Lake Area, NWT". Journal of Geochemical Exploration 28 (1-3): 409-29. http://www.sciencedirect.com/science/article/pii/0375674287900604. Retrieved 2013-08-28.
- ↑ Jaephil Cho, Steve W Martin (March 2002). "Infrared spectroscopy of glasses and polycrystals in the series xCs2S+(1−x)B2S3". Journal of non-crystalline solids 298 (2-3): 176-92. http://www.sciencedirect.com/science/article/pii/S0022309302009171. Retrieved 2013-08-28.
- ↑ P Sielicki, H Janik, A Guzman (2012). "Grain type and size of particulate matter from diesel vehicle exhausts analysed by transmission electron microscopy". Environmental Technology 33 (15). doi:10.1080/09593330.2011.646315. http://www.tandfonline.com/doi/abs/10.1080/09593330.2011.646315. Retrieved 2013-08-29.
- ↑ Mario Affatigato, Steve Feller, Allison K Schue, Sarah Blair, Dale Stentz, Garret B Smith, Dan Liss, Matt J Kelley, Cole Goater and Raghuvir Leelesagar (August 13, 2003). "Studies of oxide glass structure using laser ionization time of flight mass spectrometry". Journal of Physics: Condensed Matter 15 (31): 2323-34. http://iopscience.iop.org/0953-8984/15/31/308. Retrieved 2013-08-29.
- ↑ V. E. Villafañe, E. W. Helbling, O. Holm-Hansen (October 1995). "Spatial and temporal variability of phytoplankton biomass and taxonomic composition around Elephant Island, Antarctica, during the summers of 1990–1993". Marine Biology 123 (4): 677-86. doi:10.1007/BF00349110. http://link.springer.com/article/10.1007/BF00349110. Retrieved 2013-08-29.
- ↑ M. R. Melloch, J. M. Woodall, and E. S. Harmon, N. Otsuka, Fred H. Pollak, D. D. Nolte, R. M. Feenstra and M. A. Lutz (1995). "Low-temperature grown III-V materials". Annual Review of Materials Science 25 (1): 547-600. doi:10.1146/annurev.ms.25.080195.002555. http://www.annualreviews.org/doi/pdf/10.1146/annurev.ms.25.080195.002555. Retrieved 2013-08-29.
- ↑ M. Avella, E. Bonadies, E. Martuscelli, R Rimedio (2001). "European current standardization for plastic packaging recoverable through composting and biodegradation". Polymer Testing 20 (5): 517-21. doi:10.1016/S0142-9418(00)00068-4. http://www.sciencedirect.com/science/article/pii/S0142941800000684. Retrieved 2012-07-17.
- ↑ L.A. Dobrzański, M. Staszuk (December 2010). "PVD and CVD gradient coatings on sintered carbides and sialon tool ceramics". Journal of Achievements in Materials and Manufacturing Engineering 43 (2): 552-76. http://forsurf.pl/content/4325.pdf. Retrieved 2013-08-29.
- ↑ "Ion, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 27, 2012. Retrieved 2012-07-15.
- ↑ R. Wegmann, H.U. Schmidt, W.F. Huebner, and D.C. Boice (November 1987). "Cometary MHD and chemistry". Astronomy and Astrophysics 187 (1-2): 339-50.
- ↑ N. Cordeiro, M.N. Belgacem, A.J.D. Silvestre, C. Pascoal Neto, A. Gandini (April 1998). "Cork suberin as a new source of chemicals.: 1. Isolation and chemical characterization of its composition". International Journal of Biological Macromolecules 22 (2): 71-80. doi:10.1016/S0141-8130(97)00090-1. http://www.sciencedirect.com/science/article/pii/S0141813097000901. Retrieved 2012-07-17.
- 1 2 "Agricultural chemistry, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 12, 2012. Retrieved 2012-07-15.
- ↑ H. P. Fischer, H. P. Buser, P. Chemla, P. Huxley, W. Lutz, S. Mirza, G. M. Ramos Tombo, G. Van Lommen, V. Sipido (1994). "Synthesis and Chirality of Novel Heterocyclic Compounds Designed for Crop Protection". Bulletin des Sociétés Chimiques Belges 103 (9-10): 565-81. doi:10.1002/bscb.19941030908. http://onlinelibrary.wiley.com/doi/10.1002/bscb.19941030908/abstract. Retrieved 2012-03-16.
- ↑ B. M. Klenov and G. D. Chimitdorzhieva (October 2011). "Effect of Climate Continentality on Humus Formation and Elemental Composition of Humic Acids of Automorphic Soils of Siberia". Contemporary Problems of Ecology 4 (5): 492-6. doi:10.1134/S1995425511050067. http://www.springerlink.com/content/k8m53231t5303480/. Retrieved 2012-03-16.
- ↑ Changwen Du, Mingjiang Lei, Jianmin Zhou, Huoyan Wang, Xiaoqin Chen, Yuhua Yang (February 2011). "Effect of long‐term fertilization on the transformations of water‐extractable phosphorus in a fluvo‐aquic soil". Journal of Plant Nutrition and Soil Science 174 (1): 20-7. doi:10.1002/jpln.200900281. http://onlinelibrary.wiley.com/doi/10.1002/jpln.200900281/abstract. Retrieved 2012-03-16.
- ↑ W. Van Dokkum, R. H. De Vos, Th. Muys and J. A. Wesstra (1989). "Minerals and trace elements in total diets in the Netherlands". British Journal of Nutrition 61: 7-15. http://journals.cambridge.org/production/action/cjoGetFulltext?fulltextid=862736. Retrieved 2012-03-16.
- ↑ Murthy A. Vairavamurthy, Dusan Maletic, Shenkhe Wang, Bernard Manowitz, Timothy Eglinton, and Timothy Lyons (May 20, 1997). "Characterization of Sulfur-Containing Functional Groups in Sedimentary Humic Substances by X-ray Absorption Near-Edge Structure Spectroscopy". Energy & Fuels 11 (3): 546-53. doi:10.1021/ef960212a. http://pubs.acs.org/doi/abs/10.1021/ef960212a. Retrieved 2012-03-16.
- ↑ Peter G. Wester and Jacob de Boer, Udo A. Th. Brinkman (January 29, 1996). "Determination of Polychlorinated Terphenyls in Aquatic Biota and Sediment with Gas Chromatography/Mass Spectrometry Using Negative Chemical Ionization". Environmental Science & Technology 30 (2): 473-80. doi:10.1021/es950154s. http://pubs.acs.org/doi/abs/10.1021/es950154s?prevSearch=%2522dominant%2Bgroup%2522&searchHistoryKey=. Retrieved 2012-03-16.
- 1 2 Elyana Cuevas Montilla, Silke Hillebrand, Daniela Butschbach, Susanne Baldermann, Naoharu Watanabe, and Peter Winterhalter (August 23, 2010). "Preparative Isolation of Anthocyanins from Japanese Purple Sweet Potato (Ipomoea batatas L.) Varieties by High-Speed Countercurrent Chromatography". Journal of Agricuture & Food Chemistry 58 (18): 9899-904. doi:10.1021/jf101898j. http://pubs.acs.org/doi/abs/10.1021/jf101898j?prevSearch=%2522dominant%2Bgroup%2522&searchHistoryKey=. Retrieved 2012-03-16.
- ↑ T Mino, M.C.M. Van Loosdrecht, J.J. Heijnen (November 1998). "Microbiology and biochemistry of the enhanced biological phosphate removal process". Water Research 32 (11): 3193-207. doi:10.1016/S0043-1354(98)00129-8. http://www.sciencedirect.com/science/article/pii/S0043135498001298. Retrieved 2012-03-16.
- ↑ Hazel W. Wilkinson and R. G. Eagon (November 1971). "Type-Specific Antigens of Group B Type Ic Streptococci". Infection and Immunity 4 (5): 596-604. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC416359/pdf/iai00287-0078.pdf. Retrieved 2012-03-16.
- ↑ Gregory R. Crocetti, Philip Hugenholtz, Philip L. Bond, Andrew Schuler, Jürg Keller, David Jenkins, and Linda L. Blackall (March 2000). "Identification of Polyphosphate-Accumulating Organisms and Design of 16S rRNA-Directed Probes for Their Detection and Quantitation". Applied and Environmental Microbiology 66 (3): 1175-82. doi:10.1128/AEM.66.3.1175-1182.2000. http://aem.asm.org/content/66/3/1175.short. Retrieved 2012-03-16.
- ↑ William T. A. Harrison, Tina M. Nenoff, Thurman E. Gier, Galen D. Stucky (December 1992). "Tetrahedral-atom zincophosphate structures. Zinc diethyl phosphate, [Zn(O2P(OC2H5)2)2x, a one-dimensional inorganic" polymer""]. Inorganic Chemistry 31 (26): 5395-9. doi:10.1021/ic00052a014. http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA263040. Retrieved 2012-03-16.
- ↑ Melanie D. Tudor, Jennifer J. Becker, Peter S. White, and Michel R. Gagné (September 22, 2000). "Diastereoisomer Interconversion in Chiral BiphepPtX2 Complexes". Organometallics 19 (21): 4376-84. doi:10.1021/om000629a. http://pubs.acs.org/doi/abs/10.1021/om000629a. Retrieved 2012-03-16.
- ↑ Li Xu, Yu-Fei Ma, Kuan-Zhen Tang, Yu Tang, Wei-Sheng Liu and Min-Yu Tan (July 2008). "Preparation, Characterization and Photophysical Properties of Highly Luminescent Terbium Complexes Incorporated Into SiO2/Polymer Hybrid Material". Journal of Fluorescence 18 (3-4): 685-93. doi:10.1007/s10895-008-0344-z. http://www.springerlink.com/content/l202x50907647710/. Retrieved 2012-03-16.
- ↑ G. Frederick Wooten, Roswell Eldridge, Julius Axelrod, and Robert S. Stern (February 8, 1973). "Elevated Plasma Dopamine-β-Hydroxylase Activity in Autosomal Dominant Torsion Dystonia". New England Journal of Medicine 288 (2): 284-7. doi:10.1056/nejm197302082880604. http://www.nejm.org/doi/full/10.1056/nejm197302082880604. Retrieved 2012-03-16.
- ↑ Sandy Owega, Greg J. Evans, Robert E. Jervis, Mike Fila, Ryan D’Souza, Badi-Uz-Zaman Khan (October 2004). "Long-range sources of Toronto particulate matter (PM< sub>2.5) identified by Aerosol Laser Ablation Mass Spectrometry (LAMS)". Atmospheric Environment 38 (33): 5545-53. doi:10.1016/j.atmosenv.2004.06.034. http://www.sciencedirect.com/science/article/pii/S1352231004006405. Retrieved 2012-03-16.
- ↑ M. A. C. Hotchkis, J. E. Reiff, D. J. Vieira, F. Blönnigen, T. F. Lang, D. M. Moltz, X. Xu, and Joseph Cerny (January 1987). "Beta-delayed proton decay of 61Ge". Physical Review C 35 (1): 315-9. doi:10.1103/PhysRevC.35.315. http://prc.aps.org/abstract/PRC/v35/i1/p315_1. Retrieved 2012-03-16.
- ↑ Rebecca E. Countway, Rebecca M. Dickhut, Elizabeth A. Canuel (February 2003). "Polycyclic aromatic hydrocarbon (PAH) distributions and associations with organic matter in surface waters of the York River, VA Estuary". Organic Geochemistry 34 (2): 209-24. doi:10.1016/S0146-6380(02)00162-6. http://www.sciencedirect.com/science/article/pii/S0146638002001626. Retrieved 2012-03-16.
- ↑ Alejandro Montoya, Thanh N. Truong, and Adel F. Sarofim (August 19, 2000). "Application of Density Functional Theory to the Study of the Reaction of NO with Char-Bound Nitrogen during Combustion". The Journal of Physical Chemistry 104 (36): 8409-17. doi:10.1021/jp001045p. http://truong.hec.utah.edu/E-papers/2000-JCPA-104-8409.pdf. Retrieved 2012-03-16.
- ↑ Eric Leighton Holmes, Christopher Kelk Ingold, and Edith Hilda Ingold (1926). "CCXX.—The Nature of the Alternating Effect in Carbon Chains. Part VII. A Study of the Relative Directive Efficiencies of Oxygen and Sulphur in Aromatic Substitution". Journal of the Chemical Society: 1684-90. doi:10.1039/JR9262901684. http://pubs.rsc.org/en/content/articlepdf/1926/jr/jr9262901684. Retrieved 2012-02-22.
- ↑ E. Franklin Armstrong and Henry E. Armstrong (June 1913). "Studies on the Processes Operative in Solutions (XXX) and on Enzyme Action (XX).--The Nature of Enzymes and of their Action as Hydrolytic Agents". Proceedings of the Royal Society London B Biological Sciences 86 (568): 561-86. doi:10.1098/rspb.1913.0051. http://rspb.royalsocietypublishing.org/content/86/591/561.full.pdf. Retrieved 2012-02-22.
- ↑ "Compendium of Chemical Terminology (IUPAC "Gold Book")".
- ↑ "Functional group, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. July 8, 2012. Retrieved 2012-07-15.
- ↑ W. W. Duley, D. A. Williams (July 1981). "The infrared spectrum of interstellar dust-Surface functional groups on carbon". Royal Astronomical Society, Monthly Notices 196 (7): 269-74.
- ↑ "Organic acid, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. June 9, 2012. Retrieved 2012-07-15.
- ↑ Toshio Fujita, Junkichi Iwasa, and Corwin Hansch (December 1964). "A New Substituent Constant, π, Derived from Partition Coefficients". Journal of the American Chemical Society 86 (23): 5175-80. doi:10.1021/ja01077a028. http://tryptophan.net/chem4120/Hansch-Pi.pdf. Retrieved 2011-08-16.
- ↑ "Volatile organic compound, In: Wikipedia". San Francisco, California: Wikimedia Foundation, Inc. July 8, 2012. Retrieved 2012-07-15.
- ↑ Alexander J. MacLeod and Carl H. Snyder (May 1985). "Volatile components of two cultivars of mango from Florida". Journal of Agricultural and Food Chemistry 33 (3): 380-4. http://pubs.acs.org/doi/abs/10.1021/jf00063a015. Retrieved 2011-11-28.
- ↑ Herbst, Eric (May 12, 2005). "Chemistry of Star-Forming Regions". Journal of Physical Chemistry A 109 (18): 4017–4029. doi:10.1021/jp050461c. PMID 16833724.
- 1 2 Renyang Zheng, Li Lin, Jinglin Xie, Yuexiang Zhu and Youchang Xie (September 10, 2008). "State of Doped Phosphorus and Its Influence on the Physicochemical and Photocatalytic Properties of P-doped Titania". Journal of Physical Chemistry C 112 (39): 15502-9. doi:10.1021/jp806121m. http://pubs.acs.org/doi/abs/10.1021/jp806121m?prevSearch=%2522dominant%2Bgroup%2522&searchHistoryKey=. Retrieved 2012-03-16.
- ↑ George E. Hein (December 1976). "The science of watching and wondering". The Urban Review 9 (4): 242-8. doi:10.1007/BF02175470. http://www.springerlink.com/index/F44HW417483V1613.pdf. Retrieved 2012-03-16.
Further reading
- Dieter NH (July 1973). "A Survey of Interstellar Formaldehyde in Dust Clouds". Ap J. 183 (7): 449-68. doi:10.1086/152238. http://cdsads.u-strasbg.fr/abs/1973ApJ...183..449D.
- Price TS (1912). Per-acids and their salts. London: Longmans, Green and Co. pp. 77–8.
External links
- African Journals Online
- Bing Advanced search
- ESO - Secrets of a Dark Cloud
- GenomeNet KEGG database
- Google Books
- Google scholar Advanced Scholar Search
- Home - Gene - NCBI
- International Astronomical Union
- JSTOR
- Lycos search
- NASA/IPAC Extragalactic Database - NED
- NASA's National Space Science Data Center
- NCBI All Databases Search
- Office of Scientific & Technical Information
- PubChem Public Chemical Database
- Questia - The Online Library of Books and Journals
- SAGE journals online
- The SAO/NASA Astrophysics Data System
- Scirus for scientific information only advanced search
- SDSS Quick Look tool: SkyServer
- SIMBAD Astronomical Database
- Spacecraft Query at NASA
- SpringerLink
- Taylor & Francis Online
- WikiDoc The Living Textbook of Medicine
- Wiley Online Library Advanced Search
- Yahoo Advanced Web Search
{{Chemistry resources}}
{{Phosphate biochemistry}}