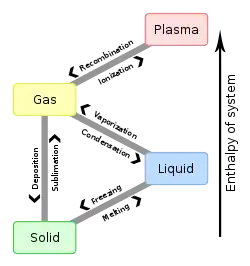
Vaporization
Vaporization is a phase change from liquid to vapor. There are two types of vaporization: evaporation and boiling.

A laboratory flask filled with pure bromine, a liquid that evaporates really fast
Evaporation is a phase change from liquid to vapour. Evaporation happens below the boiling point of a substance at a given pressure. Evaporation happens on a surface.
Boiling is also a phase change from liquid to gas. Boiling takes place when the vapor pressure of the substance is greater than or equal to the pressure of its environment. The temperature at which a substance boils is called the boiling point.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.