Vapor pressure
Vapor pressure is the pressure applied by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The vapor pressure shows how fast a liquid evaporates. A substance with a high vapor pressure at normal temperatures is often called a volatile. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules changing into a vapor also increases, so it increases the vapor pressure.
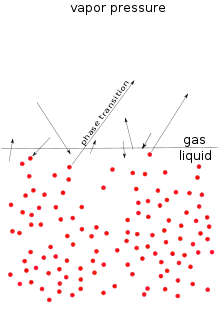
The microscopic process of evaporation and condensation at the surface of a liquid
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.