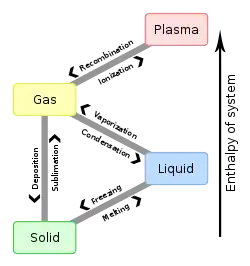
Enthalpy of fusion
Enthalpy of fusion is the measure of the energy needed to change a substance from a solid to a liquid. Extra energy is needed, more than what is obvious by the temperature scale. For example, ice at 0°C needs to absorb heat before it converts (changes) to water at 0°C. The temperature does not change, but energy is absorbed to change from solid to liquid.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.