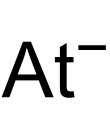| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Sodium astatide | |||
| Identifiers | |||
| Properties | |||
| NaAt | |||
| Related compounds | |||
Related compounds |
Magnesium astatide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Sodium astatide is a binary inorganic compound of sodium and astatine with the chemical formula NaAt.[1][2]
Synthesis
Sodium astatide solution has been prepared by distilling astatine from the bismuth alpha-ray target where it was prepared, dissolving in sodium bicarbonate solution, and reducing At+ and At3+ ions with ascorbic acid.[3]
Uses
Sodium astatide has been proposed for use in radiation therapy to replace 131I.[4][3]
References
- ↑ Watabe, Tadashi; Hosono, Makoto; Kinuya, Seigo; Yamada, Takahiro; Yanagida, Sachiko; Namba, Masao; Nakamura, Yoshihide (July 2021). "Manual on the proper use of sodium astatide ([211At]NaAt) injections in clinical trials for targeted alpha therapy (1st edition)". Annals of Nuclear Medicine. 35 (7): 753–766. doi:10.1007/s12149-021-01619-2. ISSN 1864-6433. PMC 8197710. PMID 33978932.
- ↑ Ball, Philip (17 March 2020). "An affinity for astatine". Chemistry World. Retrieved 16 June 2023.
- 1 2 Y. Shirakami. "Preparation of [211At]-labeled sodium astatide (NaAt) by reducing with ascorbic acid for the treatment of thyroid cancer" (PDF). RIKEN Accel. Prog. Rep. 53: 171. Retrieved 16 June 2023.
- ↑ "Breakthrough alpha-ray treatment of cancer without external radiation". EurekAlert!. Retrieved 16 June 2023.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.