 | |
| Names | |
|---|---|
| IUPAC name
Disodioarsanylsodium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.762 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Na3As | |
| Molar mass | 143.89 g·mol−1 |
| Appearance | Violet brown solid |
| Density | 2.36 g·cm−3 |
| Hydrolysis | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Toxic, releases AsH3 |
| Related compounds | |
Other anions |
Sodium nitride, sodium phosphide, sodium antimonide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sodium arsenide, also known as trisodium arsenide, is the inorganic compound of sodium and arsenic with the formula Na3As.[1] It is a dark colored solid that degrades upon contact with water or air. It is prepared by the reaction of the elements at 200–400 °C.[2] The compound is mainly of interest as exhibiting an archetypal structure. The normal pressure "sodium arsenide" phase is adopted by many alkali metal pnictides. At 3.6 gigapascals, Na3As adopts the Li3Bi structure, which is another archetypal structure.[3] Sodium arsenide is a crystalline solid used as a semiconductor and in photo optic applications. Its IUPAC name is disodioarsanylsodium.
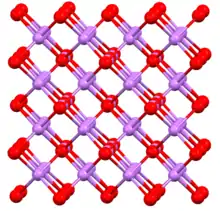
At high pressures, Na3As adopts this cubic structure (the so-called Li3Bi motif).
References
- ↑ Elements, American. "Sodium Arsenide". American Elements. Retrieved 2018-12-05.
- ↑ E. Dönges (1963). G. Brauer (ed.). Alkali Metals (2nd ed.). New York: Academic Press. p. 986.
{{cite book}}:|work=ignored (help) - ↑ Beister, Heinz Jürgen; Syassen, Karl; Klein, Jürgen (1990). "Phase Transition of Na3As under Pressure". Zeitschrift für Naturforschung B. 45 (10): 1388–1392. doi:10.1515/znb-1990-1007. S2CID 197037185.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.