| Subject classification: this is a physics resource. |
| Completion status: this resource is a stub, which means that pretty much nothing has been done yet. |
| Educational level: this is a research resource. |
| Type classification: this is an article resource. |
Electron
Electrons are negatively charged subatomic particles.
Notation Charge - Mass
Discovery of the electron
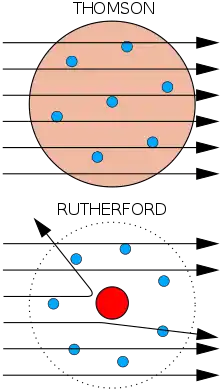
Top: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Bottom: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
The physicist J. J. Thomson measured the mass of cathode rays, showing they were made of particles, but were around 1800 times lighter than the lightest atom, hydrogen. Therefore, they were not atoms, but a new particle, the first subatomic particle to be discovered, which he originally called "corpuscle" but was later named electron, after particles postulated by George Johnstone Stoney in 1874. He also showed they were identical to particles given off by photoelectric and radioactive materials.[1] It was quickly recognized that they are the particles that carry electric currents in metal wires, and carry the negative electric charge within atoms. Thomson was given the 1906 Nobel Prize in Physics for this work. Thus he overturned the belief that atoms are the indivisible, ultimate particles of matter.[2] Thomson also incorrectly postulated that the low mass, negatively charged electrons were distributed throughout the atom in a uniform sea of positive charge. This became known as the plum pudding model.
See Also
- ↑ Thomson, J. J. (August 1901). "On bodies smaller than atoms". The Popular Science Monthly (Bonnier Corp.): 323–335. https://books.google.com/?id=3CMDAAAAMBAJ&pg=PA323. Retrieved 2009-06-21.
- ↑