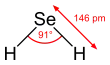Hydrogen selenide
Hydrogen selenide, also known as hydroselenic acid, selenium hydride, or selane, is a chemical compound. Its chemical formula is H2Se. It is an acid. It contains hydrogen and selenide ions.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Hydrogen selenide | |||
| Other names
Hydroselenic acid selane selenium hydride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.071 | ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2202 | ||
CompTox Dashboard (EPA) |
|||
SMILES
| |||
| Properties | |||
| H2Se | |||
| Molar mass | 80.98 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | decayed horseradish[1] | ||
| Density | 3.553 g/dm3 | ||
| Melting point | −65.73 °C (−86.31 °F; 207.42 K) | ||
| Boiling point | −41.25 °C (−42.25 °F; 231.90 K) | ||
| 0.70 g/100 mL | |||
| Solubility | soluble in CS2, phosgene | ||
| Vapor pressure | 9.5 atm (21°C)[1] | ||
| Acidity (pKa) | 3.89 | ||
| Conjugate acid | Selenonium | ||
| Conjugate base | Selenide | ||
| Structure | |||
| Bent | |||
| Hazards | |||
| EU classification | |||
| Main hazards | Extremely toxic and flammable | ||
| NFPA 704 |
| ||
| R-phrases | R23/25, R33, R50/53 | ||
| S-phrases | (S1/2), S20/21, S28, S45, S60, S61 | ||
| Flash point | flammable gas | ||
| U.S. Permissible exposure limit (PEL) |
TWA 0.05 ppm (0.2 mg/m3)[1] | ||
| Related compounds | |||
| Other anions | H2O H2S H2Te H2Po | ||
| Other cations | Na2Se Ag2Se | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Properties
Hydrogen selenide is a colorless gas that dissolves in water to make an acidic solution. It smells like rotten horseradish. It is very toxic. It burns easily, making selenium dioxide. It is similar to hydrogen sulfide, a gas that smells like rotten eggs. It is a strong reducing agent.
Preparation
Hydrogen selenide can be made by hydrolysis of aluminium selenide. This reaction also makes aluminium hydroxide. It can be made by reacting hydrogen with powdered selenium at a high temperature.
Uses
It can be used to add selenide ion to organic compounds. It can also be used to make selenium by reacting it with sulfur dioxide. This makes selenium, sulfur, and water.
References
- NIOSH Pocket Guide to Chemical Hazards. "#0336". National Institute for Occupational Safety and Health (NIOSH).