Bicarbonate
Bicarbonate is an anion (a negatively-charged ion). Its chemical formula is HCO3−. It reacts with acids to produce carbon dioxide. A sample compound is sodium bicarbonate. It forms carbonate when heated.
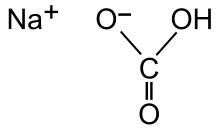
The bicarbonate anion is formed with a carbon double-bonded to an oxygen, single-bonded to a hydroxide group, which here is neutral, and single-bonded to another oxygen which requires one more electron to complete its outer shell. Here, the sodium cation provides the electron, resulting in a net -1 charge.
Bicarbonate compounds
- Sodium bicarbonate
- Potassium bicarbonate
- Calcium bicarbonate
- Ammonium bicarbonate
- Carbonic acid
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.