Azeotrope
Azeotropes are the mixtures of liquids which boil at a constant temperature. Azeotropes may be called constant boiling point mixtures or azeotropic mixtures.[1]
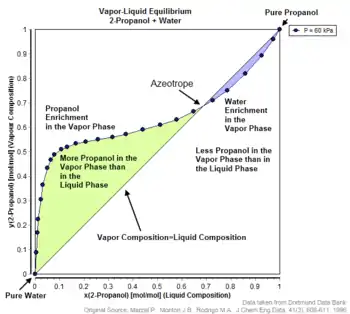
Vapour–liquid equilibrium of 2-propanol/water showing azeotropic behaviour
Their proportions cannot be altered by simple distillation.
Types
Azeotropes are classified into two types
- Minimum boiling azeotrope
- Maximum boiling azeotrope
References
- Moore, Walter J. 1962. Physical chemistry. 3rd ed Prentice-Hall, p140–142.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.