 | |
| Names | |
|---|---|
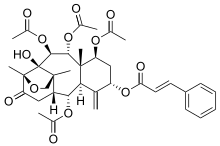
| IUPAC name
11-Hydroxy-13-oxo-12,17-epoxy-12α-tax-4(20)-ene-2α,5α,7β,9α,10β-pentayl 2,7,9,10-tetraacetate 5-[(2E)-3-phenylprop-2-enoate] | |
| Systematic IUPAC name
(1S,3aR,4R,5R,5aR,7S,9S,9aS,10R,11S,11aR)-11a-Hydroxy-1,3a,9a-trimethyl-6-methylidene-13-oxotetradecahydro-1,4-ethanobenzo[5,6]cycloocta[1,2-c]furan-5,7,9,10,11-pentayl 5,9,10,11-tetraacetate 7-[(2E)-3-phenylprop-2-enoate] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C37H44O13 | |
| Molar mass | 696.746 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Notes
- ↑ Tong J, Lu J, Zhang N, Chi H, Yamashita K, Manabe M, et al. (2009). "Effect of seven tricyclic diterpenoids from needles of Taxus media var. Hicksii on stimulus-induced superoxide generation, tyrosyl or serine/threonine phosphorylation and translocation of cytosolic compounds to the cell membrane in human neutrophils". Planta Med. 75 (8): 814–22. doi:10.1055/s-0029-1185440. PMID 19288401.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.