| TIMM8A | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | TIMM8A, DDP, DDP1, DFN1, MTS, TIM8, translocase of inner mitochondrial membrane 8 homolog A (yeast), translocase of inner mitochondrial membrane 8A | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 300356 MGI: 1353433 HomoloGene: 37878 GeneCards: TIMM8A | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Mitochondrial import inner membrane translocase subunit Tim8 A, also known as deafness-dystonia peptide or protein is an enzyme that in humans is encoded by the TIMM8A gene.[5][6][7] This translocase has similarity to yeast mitochondrial proteins that are involved in the import of metabolite transporters from the cytoplasm into the mitochondrial inner membrane. The gene is mutated in deafness-dystonia syndrome (or Mohr-Tranebjaerg syndrome; MTS/DFN-1) and it is postulated that MTS/DFN-1 is a mitochondrial disease caused by a defective mitochondrial protein import system.[7]
Structure
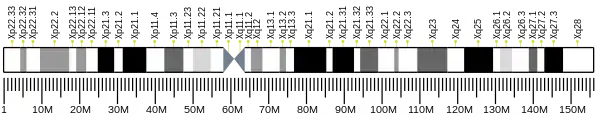
The TIMM8A gene is located on q arm of chromosome X in position 22.1 and spans 3,313 base pairs.[8] The gene produces an 11 kDa protein composed of 97 amino acids.[9][10] The structure shows resemblance to yeast translocase of the inner membrane (TIM) proteins with two conserved paired cysteine residue motifs.[11] The cysteine residues organize zinc ions for stability and control other interactions with proteins.[11]
Function
The human TIMM8A gene codes for a translocase involved in the import and insertion of hydrophobic membrane proteins from the cytoplasm into the mitochondrial inner membrane.[8] It is also required for the transfer of beta-barrel precursors from the TOM complex to the sorting and assembly machinery (SAM complex) of the outer membrane. It acts as a chaperone-like protein that protects the hydrophobic precursors from aggregation and guide them through the mitochondrial intermembrane space. The TIMM8-TIMM13 complex mediates the import of proteins such as TIMM23, SLC25A12/ARALAR1 and SLC25A13/ARALAR2, while the predominant TIMM9-TIMM10 70 kDa complex mediates the import of much more proteins. TIMM8A has been implicated as a required element in normal neurologic development.[12]
Clinical significance
Mutation of TIMM8A is associated with Mohr-Tranebjaerg syndrome or deafness-dystonia syndrome, a mitochondrial disease postulated to be associated with a defective mitochondrial protein import system. [7]Mohr-Tranebjaerg syndrome is a recessive, X-linked neurodegenerative syndrome characterized by early-onset deafness followed by progressive dystonia in adulthood, progressive sensorineural hearing loss, mental retardation, dysphagia, paranoia, and optic atrophy.[13][14] It is known to be caused by a truncation or deletion of the 11 kDa protein product of TIMM8A.[15] Defects in this gene also cause Jensen syndrome, an X-linked disease with opticoacoustic nerve atrophy and muscle weakness.[8]
A 39-year-old Japanese male patient with a nonsense mutation of the CGA codon 80 of exon 2 by TGA in the TIMM8A gene was diagnosed with deafness-dystonia syndrome. Signs and symptoms included sensorineural deafness, dystonia, blepharospasm, brisk deep tendon reflexes and personality changes. However, there were no visual or sensory disturbances. The mother was found to be a heterozygous carrier for the mutation.[13] Another patient, an 11-year-old Dutch child with a de novo missense mutation (C66W; c.233C > G) in the TIMM8A gene, was diagnosed with sensorineural hearing impairment associated with Deafness-dystonia syndrome. Signs and symptoms included hyperreflexia, dyspraxia, synkinesis, atrophy, and progressive dystonia.[16] A third patient, a 30-year-old male with Deafness-dystonia syndrome, was found to have a novel 108delG mutation in the TIMM8A gene. Signs and symptoms were generalized dystonia, scoliosis, blepharospasm, and involuntary movements of the head and neck.[17] There are many more cases of mutations in the TIMM8A gene with varying symptoms, commonly including dystonia, mental deficiency, sensorineural hearing loss, optic atrophy, and others.[18][19][20][21][22]
Interactions
TIMM8A has been shown to interact with Signal transducing adaptor molecule[11] and TIMM13.[23][24] Three copies of TIMM8A and three copies of TIMM13 assemble to form a 70 kDa TIMM8-TIMM13 Complex with heterohexamer structure in the intermembrane space.[24][12] The TIMM8-TIMM13 Complex associates with the TIM22 complex whose core is composed of TIMM22 to import and assemble inner membrane proteins.[12]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000126953 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000048007 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Jin H, Kendall E, Freeman TC, Roberts RG, Vetrie DL (November 1999). "The human family of Deafness/Dystonia peptide (DDP) related mitochondrial import proteins". Genomics. 61 (3): 259–67. doi:10.1006/geno.1999.5966. PMID 10552927.
- ↑ Jin H, May M, Tranebjaerg L, Kendall E, Fontán G, Jackson J, et al. (October 1996). "A novel X-linked gene, DDP, shows mutations in families with deafness (DFN-1), dystonia, mental deficiency and optic atrophy". Nature Genetics. 14 (2): 177–80. doi:10.1038/ng1096-177. PMID 8841189. S2CID 22065091.
- 1 2 3 "Entrez Gene: TIMM8A translocase of inner mitochondrial membrane 8 homolog A (yeast)".
- 1 2 3 "Entrez Gene: TIMM8A translocase of inner mitochondrial membrane 8A".
- ↑ Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, et al. (October 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- ↑ "TIMM8A - Mitochondrial import inner membrane translocase subunit Tim8 A". Cardiac Organellar Protein Atlas Knowledgebase (COPaKB).
- 1 2 3 Blackstone C, Roberts RG, Seeburg DP, Sheng M (May 2003). "Interaction of the deafness-dystonia protein DDP/TIMM8a with the signal transduction adaptor molecule STAM1". Biochemical and Biophysical Research Communications. 305 (2): 345–52. doi:10.1016/S0006-291X(03)00767-8. PMID 12745081.
- 1 2 3 "TIMM8A - translocase of inner mitochondrial membrane 8A, mitochondrial". UniProt.org. The UniProt Consortium.
- 1 2 Ujike H, Tanabe Y, Takehisa Y, Hayabara T, Kuroda S (June 2001). "A family with X-linked dystonia-deafness syndrome with a novel mutation of the DDP gene". Archives of Neurology. 58 (6): 1004–7. doi:10.1001/archneur.58.6.1004. PMID 11405816.
- ↑ Bauer MF, Rothbauer U, Mühlenbein N, Smith RJ, Gerbitz K, Neupert W, et al. (December 1999). "The mitochondrial TIM22 preprotein translocase is highly conserved throughout the eukaryotic kingdom". FEBS Letters. 464 (1–2): 41–7. doi:10.1016/S0014-5793(99)01665-8. PMID 10611480.
- ↑ Koehler CM, Leuenberger D, Merchant S, Renold A, Junne T, Schatz G (March 1999). "Human deafness dystonia syndrome is a mitochondrial disease". Proceedings of the National Academy of Sciences of the United States of America. 96 (5): 2141–6. Bibcode:1999PNAS...96.2141K. doi:10.1073/pnas.96.5.2141. PMC 26750. PMID 10051608.
- ↑ Tranebjaerg L, Hamel BC, Gabreels FJ, Renier WO, Van Ghelue M (June 2000). "A de novo missense mutation in a critical domain of the X-linked DDP gene causes the typical deafness-dystonia-optic atrophy syndrome". European Journal of Human Genetics. 8 (6): 464–7. doi:10.1038/sj.ejhg.5200483. PMID 10878669.
- ↑ Swerdlow RH, Wooten GF (October 2001). "A novel deafness/dystonia peptide gene mutation that causes dystonia in female carriers of Mohr-Tranebjaerg syndrome". Annals of Neurology. 50 (4): 537–40. doi:10.1002/ana.1160. PMID 11601506. S2CID 28334892.
- ↑ Dulski J, Schinwelski M, Mandat T, Pienczk-Ręcławowicz K, Sławek J (2016). "Long-Term Follow-Up with Video of a Patient with Deafness-Dystonia Syndrome Treated with DBS-GPi". Stereotactic and Functional Neurosurgery. 94 (2): 123–5. doi:10.1159/000445078. PMID 27100856. S2CID 46846035.
- ↑ Shaker M, Lorigiano TH, Vadlamudi A (June 2016). "Xq22.1 contiguous gene deletion syndrome of X-linked agammaglobulinemia and Mohr-Tranebjærg syndrome". Annals of Allergy, Asthma & Immunology. 116 (6): 578–9. doi:10.1016/j.anai.2016.03.014. PMID 27048950.
- ↑ Aguirre LA, Pérez-Bas M, Villamar M, López-Ariztegui MA, Moreno-Pelayo MA, Moreno F, del Castillo I (December 2008). "A Spanish sporadic case of deafness-dystonia (Mohr-Tranebjaerg) syndrome with a novel mutation in the gene encoding TIMM8a, a component of the mitochondrial protein translocase complexes". Neuromuscular Disorders. 18 (12): 979–81. doi:10.1016/j.nmd.2008.09.009. PMID 18952432. S2CID 27598478.
- ↑ Aguirre LA, del Castillo I, Macaya A, Medá C, Villamar M, Moreno-Pelayo MA, Moreno F (February 2006). "A novel mutation in the gene encoding TIMM8a, a component of the mitochondrial protein translocase complexes, in a Spanish familial case of deafness-dystonia (Mohr-Tranebjaerg) syndrome". American Journal of Medical Genetics. Part A. 140 (4): 392–7. doi:10.1002/ajmg.a.31079. PMID 16411215. S2CID 27887064.
- ↑ Kreisel SH, Binder J, Wöhrle JC, Krauss JK, Hofmann S, Bauer MF, et al. (October 2004). "Dystonia in the Mohr-Tranebjaerg syndrome responds to GABAergic substances". Movement Disorders. 19 (10): 1241–3. doi:10.1002/mds.20150. PMID 15390009. S2CID 31590188.
- ↑ Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, et al. (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- 1 2 Roesch K, Curran SP, Tranebjaerg L, Koehler CM (March 2002). "Human deafness dystonia syndrome is caused by a defect in assembly of the DDP1/TIMM8a-TIMM13 complex". Human Molecular Genetics. 11 (5): 477–86. doi:10.1093/hmg/11.5.477. PMID 11875042.
Further reading
- Swerdlow RH, Juel VC, Wooten GF (2003). "Dystonia with and without deafness is caused by TIMM8A mutation". Advances in Neurology. 94: 147–54. PMID 14509668.
- Tranebjaerg L, Schwartz C, Eriksen H, Andreasson S, Ponjavic V, Dahl A, et al. (April 1995). "A new X linked recessive deafness syndrome with blindness, dystonia, fractures, and mental deficiency is linked to Xq22". Journal of Medical Genetics. 32 (4): 257–63. doi:10.1136/jmg.32.4.257. PMC 1050371. PMID 7643352.
- Vorechovský I, Vetrie D, Holland J, Bentley DR, Thomas K, Zhou JN, et al. (June 1994). "Isolation of cosmid and cDNA clones in the region surrounding the BTK gene at Xq21.3-q22". Genomics. 21 (3): 517–24. doi:10.1006/geno.1994.1310. PMID 7959728.
- Wallace DC, Murdock DG (March 1999). "Mitochondria and dystonia: the movement disorder connection?". Proceedings of the National Academy of Sciences of the United States of America. 96 (5): 1817–9. Bibcode:1999PNAS...96.1817W. doi:10.1073/pnas.96.5.1817. PMC 33525. PMID 10051550.
- Nakane T, Inada Y, Ito F, Itoh N, Tazawa S, Chiba S (July 2000). "Cloning and expression of mouse deafness dystonia peptide 1 cDNA". Biochemical and Biophysical Research Communications. 273 (2): 759–64. doi:10.1006/bbrc.2000.3004. PMID 10873677.
- Paschen SA, Rothbauer U, Káldi K, Bauer MF, Neupert W, Brunner M (December 2000). "The role of the TIM8-13 complex in the import of Tim23 into mitochondria". The EMBO Journal. 19 (23): 6392–400. doi:10.1093/emboj/19.23.6392. PMC 305865. PMID 11101512.
- Rothbauer U, Hofmann S, Mühlenbein N, Paschen SA, Gerbitz KD, Neupert W, et al. (October 2001). "Role of the deafness dystonia peptide 1 (DDP1) in import of human Tim23 into the inner membrane of mitochondria". The Journal of Biological Chemistry. 276 (40): 37327–34. doi:10.1074/jbc.M105313200. PMID 11489896.
- Tranebjaerg L, Jensen PK, Van Ghelue M, Vnencak-Jones CL, Sund S, Elgjo K, et al. (December 2001). "Neuronal cell death in the visual cortex is a prominent feature of the X-linked recessive mitochondrial deafness-dystonia syndrome caused by mutations in the TIMM8a gene". Ophthalmic Genetics. 22 (4): 207–23. doi:10.1076/opge.22.4.207.2220. PMID 11803487. S2CID 19585131.
- Hofmann S, Rothbauer U, Mühlenbein N, Neupert W, Gerbitz KD, Brunner M, Bauer MF (June 2002). "The C66W mutation in the deafness dystonia peptide 1 (DDP1) affects the formation of functional DDP1.TIM13 complexes in the mitochondrial intermembrane space". The Journal of Biological Chemistry. 277 (26): 23287–93. doi:10.1074/jbc.M201154200. PMID 11956200.
External links
- The Deafness Dystonia Protein DDP and Mitochondrial Division - a free videolecture by Craig Blackstone, 2002.