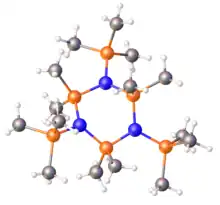
Structure of the silazane (Me3SiN)3(SiMe2)3.[1]
A silazane is a family of compounds with Si-N bonds. Usually the Si and N have organic substituents. They are analogous to siloxanes, with -NR- (R = alkyl, aryl) replacing -O-.[2]
Examples
One illustrative family of silazanes are derived from tert-butylamine, including (CH3)3SiN(H)tBu and (CH3)2Si(N(H)tBu)2.
More structurally complex is [CH3SiN(H)tBu]2(μ-N(H)tBu)2 with bridging amides.[3]
Reactions
The majority of silazanes are moisture sensitive.[4] With water they convert to silanols or siloxanes.
See also
References
- ↑ Adamson, G. W.; Daly, J. J. (1970). "Crystal and Molecular Structure of 2,2,4,4,6,6-Hexamethyl-1,3,5-tris-(trimethylsilyl)cyclotrisilazane, C15H45N3Si6, a Compound Containing a Six-Membered Ring in the 'Boat' Form". J. Chem. Soc. A: 2724–2728. doi:10.1039/j19700002724.
- ↑ "Silazanes". The IUPAC Compendium of Chemical Terminology. 2014. doi:10.1351/goldbook.S05669.
- ↑ Veith, Michael (1990). "Cage compounds with main-group metals". Chemical Reviews. 90: 3–16. doi:10.1021/cr00099a001.
- ↑ Silazane Precursors to Silicon Nitride. Defense Technical Information Center. 1984.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.