 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
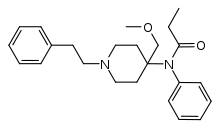
| Formula | C24H32N2O2 |
| Molar mass | 380.532 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
R-30490 (also known as 4-methoxymethylfentanyl) is an opioid analgesic related to the highly potent animal tranquilizer carfentanil, and with only slightly lower potency. It was first synthesised by a team of chemists at Janssen Pharmaceutica led by Paul Janssen, who were investigating the structure-activity relationships of the fentanyl family of drugs. R-30490 was found to be the most selective agonist for the μ-opioid receptor out of all the fentanyl analogues tested, but it has never been introduced for medical use in humans, although the closely related drug sufentanil is widely used for analgesia and anesthesia during major surgery.[2][3][4][5]
Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[6]
See also
References
- ↑ Drug Enforecement Administration, Department of Justice (February 2018). "Schedules of Controlled Substances:Temporary Placement of Fentanyl-Related Substances in Schedule I. Temporary amendment; temporary scheduling order". Federal Register. 83 (25): 5188–92. PMID 29932611.
- ↑ Cometta-Morini C, Maguire PA, Loew GH (January 1992). "Molecular determinants of mu receptor recognition for the fentanyl class of compounds". Molecular Pharmacology. 41 (1): 185–96. PMID 1310142.
- ↑ Maguire P, Tsai N, Kamal J, Cometta-Morini C, Upton C, Loew G (March 1992). "Pharmacological profiles of fentanyl analogs at mu, delta and kappa opiate receptors". European Journal of Pharmacology. 213 (2): 219–25. doi:10.1016/0014-2999(92)90685-W. PMID 1355735.
- ↑ Meert TF (January 1996). "Pharmacotherapy of opioids: present and future developments". Pharmacy World & Science. 18 (1): 1–15. doi:10.1007/BF00449683. PMID 8861825. S2CID 28280435.
- ↑ Subramanian G, Paterlini MG, Portoghese PS, Ferguson DM (February 2000). "Molecular docking reveals a novel binding site model for fentanyl at the mu-opioid receptor". Journal of Medicinal Chemistry. 43 (3): 381–91. doi:10.1021/jm9903702. PMID 10669565.
- ↑ Mounteney J, Giraudon I, Denissov G, Griffiths P (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". The International Journal on Drug Policy. 26 (7): 626–31. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.