| PRR12 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | PRR12, KIAA1205, Proline-rich 12, proline rich 12, NOC | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 616633 MGI: 2679002 HomoloGene: 18957 GeneCards: PRR12 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Proline-rich 12 (PRR12) is a protein of unknown function encoded by the gene PRR12.
Gene
The Homo sapiens PRR12 gene is 34,785 base pairs long, contains 14 exons, and is located on chromosome 19 at 19q13.33.[5] Known aliases for PRR12 include “proline rich 12” and KIAA1205.[6] Within its gene neighborhood, PRR12 is flanked by PRRG2 and SCAF1 on the sense strand and RRAS and NOSIP on the antisense strand.[5] Nitric oxide synthase interacting protein, NOSIP, regulates the activity and localization of nitric oxide synthase (endothelial and neuronal), controlling nitric oxide production.[7] Proline rich Gla, PRRG2, has a Gla domain which binds hyaluronan and is associated with proteins present in the extracellular matrix involved with cell adhesion and cell migration.[8][9] Ras-related protein R-Ras, RRAS, belongs to the Ras family and is involved in the organization of actin filaments within the cytoskeleton. SR-Related CTD-Associated [10] Factor 1, SCAF1, is thought to be involved in the splicing of precursor mRNA.[11]
 Location of PRR12 on chromosome 19
Location of PRR12 on chromosome 19 PRR12 gene neighborhood
PRR12 gene neighborhood
Promoter
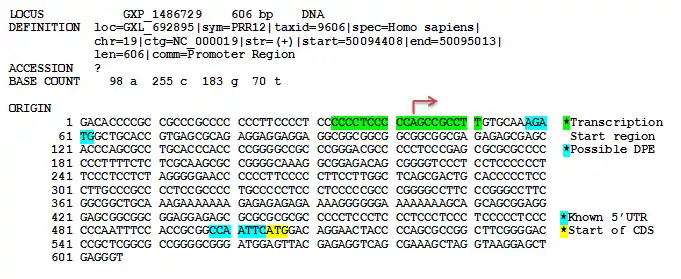
The promoter region of PRR12 was predicted using ElDorado at Genomatix.[12] The region starts at position 50094408 and ends at 50095013 of chromosome 19. This promoter set is conserved in the macaque, mouse, rat, horse, cow, pig, dog and zebrafish. No recognizable TATA box, B recognition element (BRE), or CAAT box was found upstream of the predicted transcription start region. Because no clear TATA box was found, it is possible that PRR12 is regulated by a TATA-less promoter containing a downstream promoter element (DPE). However, the predicted DPE is only 15bp downstream of the transcription start region instead of the typical +25 to +32 base pairs. More research will be required to expand the 5’ UTR of the PRR12 transcript in order to confirm where the correct promoter region is located.
Transcript
mRNA sequence
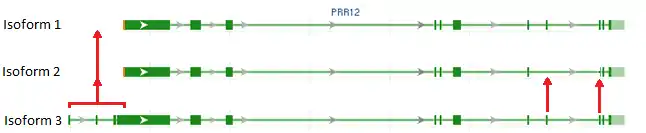
The PRR12 mRNA transcript is 6960 base pairs long and contains several short sequence repeats. The Homo sapiens PRR12 has three isoforms with isoform 3 containing roughly one thousand more amino acid residues than the other isoforms.[13] No 5’ UTR is given in the NCBI records for the Homo sapiens PRR12 transcript. However, 7 base pairs of the 5’ UTR have been determined in the Papio anubis ortholog. The 3’ UTR is 852 base pairs long.[14]
Expression
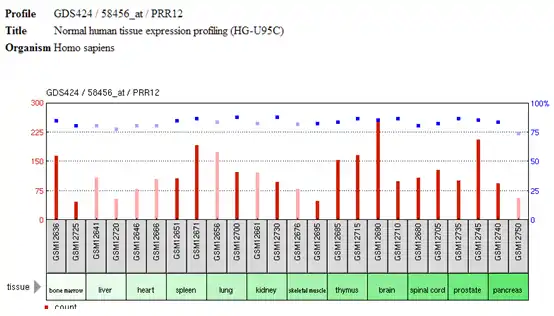
The gene is moderately expressed at even levels in a wide variety of tissue types.[6]
Protein
The PRR12 transcript encodes a protein that is 2036 residues long. It has a molecular weight of 211.1 kdal and an isoelectric point around 7.728.[15] A number of bioinformatics databases have also predicted PRR12 to be a soluble protein with no transmembrane domains.[15][16][17] Jianping Chen lists PRR12 as an "extremely vulnerable protein".[18] These proteins have regions rich in amino acids that are “poor protectors” of hydrogen bonds along the backbone of the protein, inhibiting the ability of these proteins to fold properly and allowing the possibility of protein aggregation. Residues such as G, A, S, Y, and P are listed as poor protectors and PRR12 is rich in both proline and glycine.[15][18] Many of the proline residues are positioned consecutively in regions of low complexity. These regions may give this protein interesting secondary structure as a cluster of proline can form a polyproline helix.[19] PRR12 contains a possible nuclear import signal starting at P1794. A typical nuclear localization sequence would have the following residues: P-P-K-K-K-R-K-V.[20] PRR12 contains a DUF4211 domain starting at V1836 that shows homology to the pfam13926 domain.[21] This domain is well conserved in PRR12 orthologs. PRR12 also contains well conserved AT-hook binding regions at P1168 and G1202. These regions allow proteins to bind DNA, further supporting the localization of PRR12 to the nucleus.

Conservation
Paralogs
The glutamine and serine-rich protein 1 (QSER1) is the only closely related paralog to PRR12 (NCBI accession: EAW68214). QSER1 has no known function and, like PRR12, it contains DUF4211 and a nuclear localization signal. QSER1 does not contain the AT binding regions or Epstein-Barr virus antigen that is found in PRR12.
Orthologs
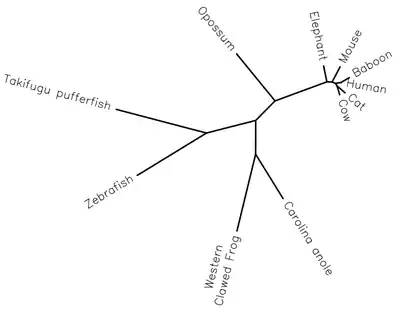
The most distant relative found through BLAST with a significant similarity to PRR12 is the fish Danio rerio. Orthologs were found in fish, amphibians, reptiles, and other mammals. While no PRR12 orthologs were found in birds, birds did have orthologs to the QSER1, which is a close paralog to human PRR12.[13]

Clinical importance
One study on the Epstein–Barr virus found close homology between a proline rich region in PRR12 and a 65 amino acid long region at the terminal end of EBNA-2 (a nuclear antigen of the virus).[22] This Epstein-Barr virus antigen is associated with autoimmune systemic connective tissue diseases (CTD) including systemic lupus erythematosus (SLE), primary Sjögren syndrome (SS), rheumatoid arthritis (RA), systemic sclerosis (SSc), and secondary SS.[22] PRR12 is not only proline rich, but it is also rich in glycine, suggesting that there might be a relationship to collagen which is also proline and glycine rich. A relationship between the two might be an explanation for the appearance of autoimmune CTDs after infection of EBV. However, glycine and proline residues in collagen generally follow a G-P-X or G-X-HydroxyP motif, which does not significantly occur in PRR12.
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000126464 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000046574 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- 1 2 "PRR12 proline rich 12 [Homo sapiens (human)] - Gene - NCBI". Ncbi.nlm.nih.gov. Retrieved 2014-01-26.
- 1 2 "PRR12 Gene - GeneCards | PRR12 Protein | PRR12 Antibody". GeneCards. Retrieved 2014-01-26.
- ↑ "NOSIP". Retrieved 6 November 2014.
- ↑ "PRRG2". Retrieved 6 November 2014.
- ↑ "GLA". Retrieved 6 November 2014.
- ↑ "RRAS". Retrieved 6 November 2014.
- ↑ "SCAF1". Retrieved 6 November 2014.
- ↑ "Genomes and Annotation: ElDorado".
- 1 2 "Proline-rich protein 12 - Homo sapiens (Human)". Uniprot.org. Retrieved 2014-01-26.
- ↑ "Homo sapiens proline rich 12 (PRR12), mRNA". Retrieved 2014-11-06.
- 1 2 3 http://seqtool.sdsc.edu/CGI/BW.cgi#%5B%5D!
- ↑ "EMBOSS: antigenic". Emboss.bioinformatics.nl. Retrieved 2014-01-26.
- ↑ "PSORT II Prediction". Psort.hgc.jp. 1999-11-24. Retrieved 2014-01-26.
- 1 2 Chen, Jianping (2009). Molecular Basis of Gene Dosage Sensitivity (Ph.D.). Rice University. ISBN 9781109217346. ProQuest 304989828.
- ↑ Williamson MP (Jan 15, 1994). "The structure and function of proline-rich regions in proteins". The Biochemical Journal. 297 (Pt 2): 249–60. doi:10.1042/bj2970249. PMC 1137821. PMID 8297327.
- ↑ Bruce Alberts; et al. (2008). Molecular biology of the cell (5th ed.). New York: Garland Science. ISBN 978-0-8153-4111-6.
- ↑ "PRR12 protein [Homo sapiens] - Protein - NCBI". Ncbi.nlm.nih.gov. 2013-08-12. Retrieved 2014-01-26.
- 1 2 Yamazaki M, Kitamura R, Kusano S, Eda H, Sato S, Okawa-Takatsuji M, Aotsuka S, Yanagi K (March 2005). "Elevated immunoglobulin G antibodies to the proline-rich amino-terminal region of Epstein-Barr virus nuclear antigen-2 in sera from patients with systemic connective tissue diseases and from a subgroup of Sjögren's syndrome patients with pulmonary involvements". Clinical & Experimental Immunology. 139 (3): 558–568. doi:10.1111/j.1365-2249.2004.02704.x. PMC 1809310. PMID 15730403.