 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| 1847301 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.790 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
| UN number | 3295 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H18 | |
| Molar mass | 138.254 g·mol−1 |
| Appearance | colorless liquids |
| Boiling point | 167.2 – 168 °C (cis) 164 °C (trans) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
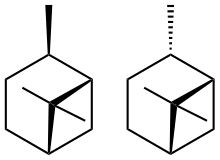
Pinane is a set of isomeric terpenes. Existing as chiral cis and trans isomers, they arise from the hydrogenation of pinene. Both isomers undergo reaction with air to give 2-pinane hydroperoxides, also with chiral cis and trans isomers.[1] Partial reduction of these isomers gives 2-pinanol.[2]
References
- ↑ Erman, Mark B.; Kane, Bernard J. (2008). "Chemistry Around Pinene and Pinane: A Facile Synthesis of Cyclobutanes and Oxatricyclo‐Derivative of Pinane from cis‐ and trans‐Pinanols". Chemistry & Biodiversity. 5 (6): 910–919. doi:10.1002/cbdv.200890104. PMID 18618388. S2CID 24782774.
- ↑ Eggersdorfer, Manfred (2000). "Terpenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_205. ISBN 978-3527306732.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.