 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
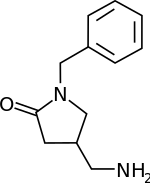
| Formula | C12H16N2O |
| Molar mass | 204.273 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Nebracetam is an investigational drug of the racetam family that is a M1 acetylcholine receptor agonist in rats.[1] Based on a human leukemic T cell experiment in 1991, it is believed to act as an agonist for human M1-muscarinic receptors.[2] It is also believed to act as a nootropic, like many other racetam drugs.[3] A chemoenzymatic method of synthesis was reported in 2008.[4] As of 2023, human trials have not yet been conducted.
See also
References
- ↑ Takeo S, Hayashi H, Miyake K, Takagi K, Tadokoro M, Takagi N, Oshikawa S (June 1997). "Effects of delayed treatment with nebracetam on neurotransmitters in brain regions after microsphere embolism in rats". British Journal of Pharmacology. 121 (3): 477–84. doi:10.1038/sj.bjp.0701161. PMC 1564714. PMID 9179389.
- ↑ Kitamura, Y.; Kaneda, T.; Nomura, Y. (January 1991). "Effects of nebracetam (WEB 1881 FU), a novel nootropic, as a M1-muscarinic agonist". Japanese Journal of Pharmacology. 55 (1): 177–180. doi:10.1254/jjp.55.177. ISSN 0021-5198. PMID 2041225.
- ↑ Gabryel, B.; Pudełko, A.; Trzeciak, H. I.; Cieślik, P. (2000). "Effect of nebracetam on content of high-energy phosphates and morphometry of rat astrocytes in vitro. Comparison with piracetam". Acta Poloniae Pharmaceutica. 57 (4): 289–298. ISSN 0001-6837. PMID 11126618.
- ↑ Yamashita, Sho; Mase, Nobuyuki; Takabe, Kunihiko (2008-09-22). "Chemoenzymatic total synthesis and determination of the absolute configuration of (S)-nebracetam". Tetrahedron: Asymmetry. 19 (18): 2115–2118. doi:10.1016/j.tetasy.2008.09.004. ISSN 0957-4166.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.