 | |
| Names | |
|---|---|
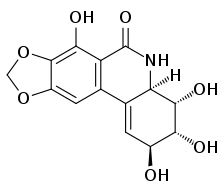
| Preferred IUPAC name
(2S,3R,4S,4aR)-2,3,4,7-Tetrahydroxy-3,4,4a,5-tetrahydro-9H-[1,3]dioxolo[4,5-j]phenanthridin-6(2H)-one | |
| Other names
BRN 1087400, Lycoricidin-A, Lycoricidinol, NSC 266535 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.214.093 |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H13NO7 | |
| Molar mass | 307.258 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Narciclasine is a toxic alkaloid found in various Amaryllidaceae species.[1]
References
- ↑ Kornienko A, Evidente A (2008). "Chemistry, biology, and medicinal potential of narciclasine and its congeners". Chem Rev. 108 (6): 1982–2014. doi:10.1021/cr078198u. PMC 2856661. PMID 18489166.
Bibliography
- Gwendoline Van Goietsenoven; Véronique Mathieu; Florence Lefranc; Alexander Kornienko; Antonio Evidente; Robert Kiss (March 2013). "Narciclasine as well as other Amaryllidaceae Isocarbostyrils are Promising GTP-ase Targeting Agents against Brain Cancers". Medicinal Research Reviews. 33 (2): 439–455. doi:10.1002/med.21253.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.