 | |
| Names | |
|---|---|
| IUPAC name
(2R)-2-[[(2R)-5-(Diaminomethylideneamino)-2-[(2,3-dihydroxybenzoyl)amino]pentanoyl]amino]-5-[(2,3-dihydroxybenzoyl)oxy-formylamino]pentanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C26H32N6O11 | |
| Molar mass | 604.573 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Mirubactin is a siderophore produced by the bacterium Actinosynnema mirum. A. mirum was first isolated from the Raritan River in New Jersey in 1976, and its full genome sequence was published in 2009.[1] In 2012, mirubactin was isolated and characterized, and the biosynthesis was connected with the gene cluster Amir_2714-Amir_2728, since renamed mrbA-mrbO.[2]
Biosynthesis
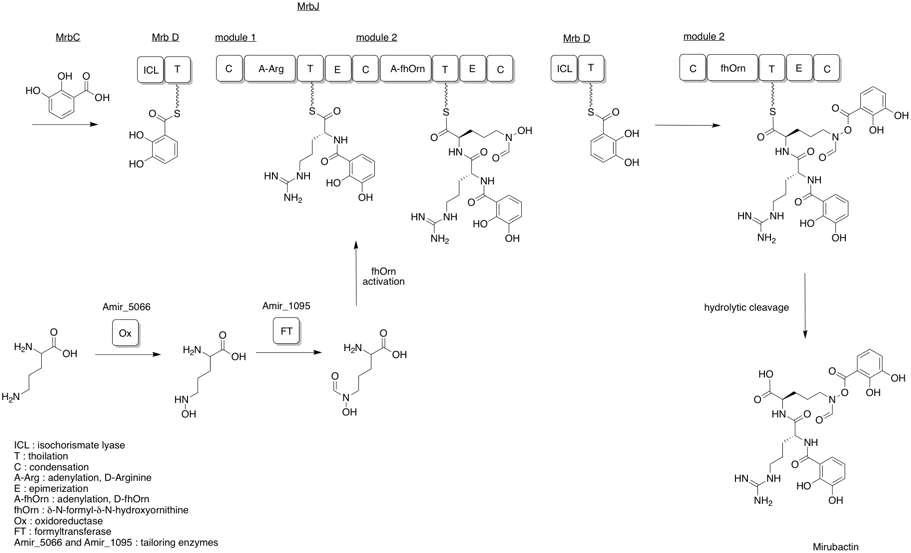
The largest gene in the mrbA-mrbO cluster, mrbJ, encodes a two-module nonribosomal peptide synthetase. Mirubactin assembly uses two nonproteinogenic units, 2,3-dihydroxybenzoic acid (2,3-DHB) and δ-N-formyl-δ-N-hydroxyornithine (fhOrn). MrbC and MrbD work to activate and incorporate 2,3-DHB into the nonribosomal peptide synthesis. The 2,3-DHB is incorporated on both the N- and C- termini, and is attached to the δ-N-hydroxyl group of fhOrn to form a O-acyl-hydroxamic acid ester. At the start of the assembly, 2,3-DHB is activated by MrbC and subsequently passed to MrbD, where it is attached to the arginine in the first module by MrbJ. The second module of MrbJ incorporates fhOrn, which is generated by the tailoring enzymes Amir_5066 and Amir_1095. The second addition of 2,3-DHB is followed by a hydrolytic cleavage, releasing the siderophore.
References
- ↑ Land, Miriam; Lapidus, Alla; Mayilraj, Shanmugam; Chen, Feng; Copeland, Alex; Rio, Tijana Glavina Del; Nolan, Matt; Lucas, Susan; Tice, Hope (2009-07-20). "Complete genome sequence of Actinosynnema mirum type strain (101T)". Standards in Genomic Sciences. 1 (1): 46–53. doi:10.4056/sigs.21137. ISSN 1944-3277. PMC 3035213. PMID 21304636.
- ↑ Giessen, Tobias; Franke, Kamila; Knappe, Thomas; Kraas, Femke; Bosello, Mattia; Xie, Xiulan; Linne, Uwe; Marahiel, Mohamed (2012). "Isolation, Structure Elucidation, and Biosynthesis of an Unusual Hydroxyamin Acid Ester-Containing Siderophore from Actinosynnema mirum". Journal of Natural Products. 75 (5): 905–914. doi:10.1021/np300046k. PMID 22578145.