 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| CH3BiCl2 | |
| Molar mass | 294.92 g·mol−1 |
| Appearance | yellow solid |
| Density | 4.009 g/cm3 |
| Melting point | 242 °C (468 °F; 515 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Methylbismuth dichloride is the organobismuth compound with the formula CH3BiCl2. It is a pale yellow solid. The compound can be prepared in two steps from diphenylbismuth chloride, first by methylation with methylmagnesium chloride. Treatment of the resulting methyldiphenylbismuthine with hydrogen chloride cleaves the two phenyl-bismuth bonds.
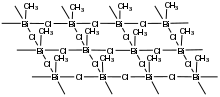
The compound adopts a polymeric structure wherein each square pyramidal Bi center is bound to four chloride ligands and an apical methyl group. The bismuth centers are interconnected by doubly bridged chloride centers.[1]
References
- ↑ Althaus, Henrik; Breunig, Hans Joachim; Lork, Enno (2001). "Syntheses and Chemistry of Methylantimony and Methylbismuth Dihalides: An Extended Two-Dimensional Framework in the Crystal Structure of CH3BiCl2 and Molecular Units in the Structures of [CH3ECl2(2,2'-bipyridine)] (E = Sb, Bi)". Organometallics. 20 (3): 586–589. doi:10.1021/om000749i.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.