 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
PubChem CID |
|
| |
| |
| Properties | |
| C24H30O8 | |
| Molar mass | 446.496 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
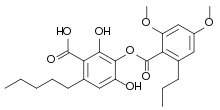
Merochlorophaeic acid is a depside with the molecular formula C24H30O8 which has been isolated from the lichen Cladonia merochlorophaea.[1][2][3][4]
References
- ↑ "Merochlorophaeic acid". pubchem.ncbi.nlm.nih.gov.
- ↑ Shibata, Shoji; Chiang, Hsüch-Ching (February 1965). "The structures of cryptochlorophaeic acid and merochlorophaeic acid". Phytochemistry. 4 (1): 133–139. doi:10.1016/S0031-9422(00)86155-5.
- ↑ Hawksworth, David L. Ascomycete Systematics: Problems and Perspectives in the Nineties. Springer. p. 157. ISBN 978-1-4757-9290-4.
- ↑ Elix, Ja; Norfolk, S (1975). "Synthesis of meta-Divarinol and Olivetol depsides". Australian Journal of Chemistry. 28 (2): 399. doi:10.1071/CH9750399.
Further reading
- Symposium, International Association for Lichenology. Progress and Problems in Lichenology in the Nineties: Proceedings of the Third Symposium of the International Association for Lichenology (IAL3) Held at the University of Salzburg, Salzburg, Austria on 1.-7. September 1996. J. Cramer. p. 48. ISBN 978-3-443-58047-6.
- Reinhold, Leonora; Liwschitz, Yecheskel. Progress in Phytochemistry. Pergamon Press. p. 236.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.