 | |
| Names | |
|---|---|
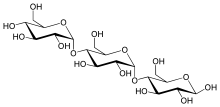
| IUPAC name
α-D-Glucopyranosyl-(1→4)-α-D-glucopyranosyl-(1→4)-D-glucopyranose | |
| Systematic IUPAC name
(2R,3S,4S,5S,6R)-2-[(2R,3R,4R,5R,6R)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2R,3S,4S,5R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.012.886 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H32O16 | |
| Molar mass | 504.438 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Maltotriose is a trisaccharide (three-part sugar) consisting of three glucose molecules linked with α-1,4 glycosidic bonds.[1]
It is most commonly produced by the digestive enzyme alpha-amylase (a common enzyme in human saliva) on amylose in starch. The creation of both maltotriose and maltose during this process is due to the random manner in which alpha amylase hydrolyses α-1,4 glycosidic bonds.
It is the shortest chain oligosaccharide that can be classified as maltodextrin.
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.