 | |
| Clinical data | |
|---|---|
| Other names | Lu AA-33810 |
| ATC code |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
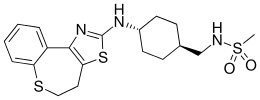
| Formula | C19H25N3O2S3 |
| Molar mass | 423.61 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Lu AA-33810 is a drug developed by Lundbeck, which acts as a potent and highly selective antagonist for the Neuropeptide Y receptor Y5, with a Ki of 1.5nM and around 3300x selectivity over the related Y1, Y2 and Y4 receptors. In animal studies it produced anorectic, antidepressant and anxiolytic effects, and further research is now being conducted into its possible medical application in the treatment of eating disorders.[1]
References
- ↑ Walker MW, Wolinsky TD, Jubian V, Chandrasena G, Zhong H, Huang X, Miller S, Hegde LG, Marsteller DA, Marzabadi MR, Papp M, Overstreet DH, Gerald CP, Craig DA (March 2009). "The novel neuropeptide Y Y5 receptor antagonist Lu AA33810 (N-([trans-4-[(4,5-dihydro[1]benzothiepino[5,4-d]thiazol-2-yl)amino]cyclohexyl]methyl)-methanesulfonamide) exerts anxiolytic- and antidepressant-like effects in rat models of stress sensitivity". The Journal of Pharmacology and Experimental Therapeutics. 328 (3): 900–11. doi:10.1124/jpet.108.144634. PMID 19098165. S2CID 20295744.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.