| Krapcho decarboxylation | |
|---|---|
| Named after | A. Paul Krapcho |
| Reaction type | Substitution reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000507 |
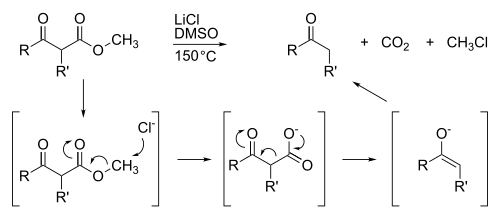
The Krapcho decarboxylation is the chemical reaction of esters with halide anions. The ester must contain an electron-withdrawing group in the beta position, such as β-ketoesters, malonic esters, α-cyanoesters, or α-sulfonylesters. It works best with methyl esters, since methyl groups are more susceptible to SN2-reaction than are larger alkyl ester substituents, such as ethyl or propyl groups. The byproducts of this decarbomethoxylation are chloromethane and CO2. They are lost as gases, which helps drive the reaction.
The Krapcho decarboxylation is a useful way to manipulate malonic esters because it cleaves only one of the two ester groups. The apparent alternative, base hydrolysis followed by decarboxylation, requires a subsequent step to regenerate the ester.[1][2][3][4]
References
- ↑ Flynn, Daniel L.; Becker, Daniel P.; Nosal, Roger; Zabrowski, Daniel L. (1992-11-24). "Use of atom-transfer radical cyclizations as an efficient entry into a new "serotonergic" azanoradamantane". Tetrahedron Letters. 33 (48): 7283–7286. doi:10.1016/S0040-4039(00)60166-1. ISSN 0040-4039.
- ↑ Krapcho, A. Paul; Weimaster, J. F.; Eldridge, J. M.; Jahngen, E. G. E.; Lovey, A. J.; Stephens, W. P. (1978-01-01). "Synthetic applications and mechanism studies of the decarbalkoxylations of geminal diesters and related systems effected in dimethyl sulfoxide by water and/or by water with added salts". The Journal of Organic Chemistry. 43 (1): 138–147. doi:10.1021/jo00395a032. ISSN 0022-3263.
- ↑ Krapcho, A. Paul; Jahngen, E. G. E.; Lovey, A. J.; Short, Franklin W. (1974-01-01). "Decarbalkoxylations of geminal diesters and β-keto esters in wet dimethyl sulfoxide. Effect of added sodium chloride on the decarbalkoxylation rates of mono- and di-substituted Malonate esters". Tetrahedron Letters. 15 (13): 1091–1094. doi:10.1016/S0040-4039(01)82414-X. ISSN 0040-4039.
- ↑ Krapcho, A. Paul; Glynn, Gary A.; Grenon, Brian J. (1967-01-01). "The decarbethoxylation of geminal dicarbethoxy compounds". Tetrahedron Letters. 8 (3): 215–217. doi:10.1016/S0040-4039(00)90519-7. ISSN 0040-4039. PMID 6037875.