Immunoadsorption is a procedure that removes specific blood group antibodies from the blood.[1] It is needed to remove the antibodies against pathogenic antibodies.[2][3][4]
The procedure generally takes about three to four hours.[5]
Immunoadsorption was developed in the 1990s as a method of extracorporeal removal of molecules from the blood, in particular molecules of the immune system.
Different number of devices/columns exist on the market, each with a different active component to which the molecule of interest attaches, allowing for selectivity in the molecules of interest.
Immunoadsorption may be used as an alternative to plasma exchange in certain conditions.[6] Evidence of benefit is lacking in those with kidney problems. Concerns include that it is expensive.[7]
Procedure
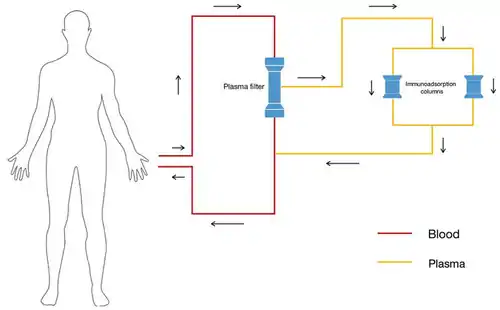
Dual column system
Blood first passes to plasma filter. Plasma then passes on to immunoadsorption column before returning to patient. As the plasma is passing through one column, the second column is being regenerated. Once the first column is saturated the flow switches to the second column while the first is then regenerated.
-1st step: the separation of plasma from the blood cells
-2nd step: the immunoadsorption column
Treatment prescriptions for immunoadsorption are based on plasma volumes with different recommendations for each condition and depending on the condition being treated, sessions can be daily or intermittent.
The therapy
Immunoadsorption could be used in various autoimmune-mediated neurological diseases in order to remove autoimmune antibodies and other pathological constituents from the patients blood
It is increasingly recognized as a more specific alternative and generally appreciated for its potentially advantageous safety profile.[7]
Immunoadsorption is also used in kidney transplantation for either the preparation of the ABO-incompatible or the highly sensitized kidney transplant candidate before transplantation, or the treatment of antibody-mediated rejection after transplantation.[8]
Indication
The most frequently encountered complication of immunoadsorption is an allergic reaction to the filter or adsorption column. Medication may be given before the procedure to minimize the risk.
Other side effects during the treatment could be dizziness, nausea or feeling cold.[5]
The usage of immunoadsorption as medical procedure is still limited in some countries of the world, especially in Northern America. The additional costs for immunoadsorption are balanced by the reduced length of stay time as well as the reduced need of plasma substituting solutions and handling of side effects.[9]
References
- ↑ Koziolek, Michael J; Tampe, Desiree; Bähr, Matthias; Dihazi, Hassan; Jung, Klaus; Fitzner, Dirk; Klingel, Reinhard; Müller, Gerhard A; Kitze, Bernd (2012). "Immunoadsorption therapy in patients with multiple sclerosis with steroid-refractory optical neuritis". Journal of Neuroinflammation. 9: 80. doi:10.1186/1742-2094-9-80. PMC 3418188. PMID 22537481.
- ↑ Ikeda, Uichi; Kasai, Hiroki; Izawa, Atsushi; Koyama, Jun; Yazaki, Yoshikazu; Takahashi, Masafumi; Higuchi, Makoto; Koh, Chang-Sung; Yamamoto, Keiji (2008). "Immunoadsorption Therapy for Patients with Dilated Cardiomyopathy and Heart Failure". Current Cardiology Reviews. 4 (3): 219–22. doi:10.2174/157340308785160534. PMC 2780823. PMID 19936198.
- ↑ Hohenstein, B.; Bornstein, S.R.; Aringer, M. (2013). "Immunoadsorption for connective tissue disease". Atherosclerosis Supplements. 14 (1): 185–9. doi:10.1016/j.atherosclerosissup.2012.10.034. PMID 23357163.
- ↑ Terman, Davids.; Buffaloe, George; Cook, Gary; Sullivan, Michael; Mattioli, Carlos; Tillquist, Richard; Carlos Ayus, Juan (1979). "Extracorporeal Immunoadsorption: Initial Experience in Human Systemic Lupus Erythematosus". The Lancet. 314 (8147): 824–827. doi:10.1016/S0140-6736(79)92177-9. PMID 90920. S2CID 45206606.
- 1 2 Yadav, Satyen. "University Hospital Birmingham, Immunoasorption" (PDF). Archived from the original (PDF) on 2011-11-09. Retrieved 2021-06-29.
- ↑ Kronbichler, A; Brezina, B; Quintana, LF; Jayne, DR (January 2016). "Efficacy of plasma exchange and immunoadsorption in systemic lupus erythematosus and antiphospholipid syndrome: A systematic review". Autoimmunity Reviews. 15 (1): 38–49. doi:10.1016/j.autrev.2015.08.010. PMID 26318215.
- 1 2 Dorst, Johannes; Fillies, Frank; Dreyhaupt, Jens; Senel, Makbule; Tumani, Hayrettin (September 2020). "Safety and Tolerability of Plasma Exchange and Immunoadsorption in Neuroinflammatory Diseases". Journal of Clinical Medicine. 9 (9): 2874. doi:10.3390/jcm9092874. PMC 7565027. PMID 32899499.
- ↑ Schwenger, V.; Morath, C. (2010-08-01). "Immunoadsorption in nephrology and kidney transplantation". Nephrology Dialysis Transplantation. 25 (8): 2407–2413. doi:10.1093/ndt/gfq264. ISSN 0931-0509. PMID 20472578.
- ↑ Schneider-Gold, Christiane; Krenzer, Marco; Klinker, Erdmute; Mansouri-Thalegani, Behrouz; Müllges, Wolfgang; Toyka, Klaus V.; Gold, Ralf (July 2016). "Immunoadsorption versus plasma exchange versus combination for treatment of myasthenic deterioration". Therapeutic Advances in Neurological Disorders. 9 (4): 297–303. doi:10.1177/1756285616637046. ISSN 1756-2864. PMC 4916519. PMID 27366236.
Further reading
- Goralczyk, Armin D.; Obed, Aiman; Schnitzbauer, Andreas; Doenecke, Axel; Tsui, Tung Yu; Scherer, Marcus N.; Ramadori, Giuliano; Lorf, Thomas (2009). "Adult Living Donor Liver Transplantation with ABO-Incompatible Grafts: A German Single Center Experience". Journal of Transplantation. 2009: 1–8. doi:10.1155/2009/759581. PMC 2817542. PMID 20148072.
- Schwenger, V.; Morath, C. (2010). "Immunoadsorption in nephrology and kidney transplantation". Nephrology Dialysis Transplantation. 25 (8): 2407–2413. doi:10.1093/ndt/gfq264. PMID 20472578.