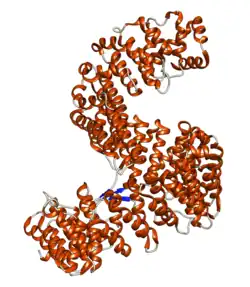
Importin-5 is a protein that in humans is encoded by the IPO5 gene.[5][6][7] The protein encoded by this gene is a member of the importin beta family. Structurally, the protein adopts the shape of a right hand solenoid and is composed of 24 HEAT repeats.[8]
Function
Nuclear transport, a signal- and energy-dependent process, takes place through nuclear pore complexes embedded in the nuclear envelope. The import of proteins containing a nuclear localization signal (NLS) requires the NLS import receptor, a heterodimer of importin alpha and beta subunits also known as karyopherins. Importin alpha binds the NLS-containing cargo in the cytoplasm and importin beta docks the complex at the cytoplasmic side of the nuclear pore complex. In the presence of nucleoside triphosphates and the small GTP binding protein Ran, the complex moves into the nuclear pore complex and the importin subunits dissociate. Importin alpha enters the nucleoplasm with its passenger protein and importin beta remains at the pore. Interactions between importin beta and the FG repeats of nucleoporins are essential in translocation through the pore complex.[9]
IPO5 facilitates cytoplasmic polyadenylation element-binding protein (CPEB)3 translocation by binding to RRM1 motif of CPEB3 in neurons. NMDAR signaling increases RanBP1 expression and reduces the level of cytoplasmic GTP-bound Ran. These changes enhance CPEB3–IPO5 interaction, which consequently accelerates the nuclear import of CPEB3 and promotes its nuclear function.[10]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000065150 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000030662 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Yaseen NR, Blobel G (April 1997). "Cloning and characterization of human karyopherin beta3". Proceedings of the National Academy of Sciences of the United States of America. 94 (9): 4451–6. Bibcode:1997PNAS...94.4451Y. doi:10.1073/pnas.94.9.4451. PMC 20743. PMID 9114010.
- ↑ Deane R, Schäfer W, Zimmermann HP, Mueller L, Görlich D, Prehn S, et al. (September 1997). "Ran-binding protein 5 (RanBP5) is related to the nuclear transport factor importin-beta but interacts differently with RanBP1". Molecular and Cellular Biology. 17 (9): 5087–96. doi:10.1128/mcb.17.9.5087. PMC 232359. PMID 9271386.
- ↑ Deng T, Engelhardt OG, Thomas B, Akoulitchev AV, Brownlee GG, Fodor E (December 2006). "Role of ran binding protein 5 in nuclear import and assembly of the influenza virus RNA polymerase complex". Journal of Virology. 80 (24): 11911–9. doi:10.1128/JVI.01565-06. PMC 1676300. PMID 17005651.
- ↑ Swale C, Da Costa B, Sedano L, Garzoni F, McCarthy AA, Berger I, et al. (May 2020). "X-ray Structure of the Human Karyopherin RanBP5, an Essential Factor for Influenza Polymerase Nuclear Trafficking" (PDF). Journal of Molecular Biology. 432 (10): 3353–3359. doi:10.1016/j.jmb.2020.03.021. PMID 32222384. S2CID 219670671.
- ↑ "Entrez Gene: RANBP5 RAN binding protein 5".
- ↑ Chao HW, Lai YT, Lu YL, Lin CL, Mai W, Huang YS (September 2012). "NMDAR signaling facilitates the IPO5-mediated nuclear import of CPEB3". Nucleic Acids Research. 40 (17): 8484–98. doi:10.1093/nar/gks598. PMC 3458550. PMID 22730302.
Further reading
- Bukrinsky MI, Haffar OK (December 1997). "HIV-1 nuclear import: in search of a leader". Frontiers in Bioscience. 2 (4): d578-87. doi:10.2741/A213. PMID 9366553.
- Bukrinsky MI, Haffar OK (March 1998). "HIV-1 nuclear import: matrix protein is back on center stage, this time together with Vpr". Molecular Medicine. 4 (3): 138–43. doi:10.1007/BF03401911. PMC 2230352. PMID 9562972.
- Christophe D, Christophe-Hobertus C, Pichon B (May 2000). "Nuclear targeting of proteins: how many different signals?". Cellular Signalling. 12 (5): 337–41. doi:10.1016/S0898-6568(00)00077-2. PMID 10822175.
- Chook YM, Blobel G (December 2001). "Karyopherins and nuclear import". Current Opinion in Structural Biology. 11 (6): 703–15. doi:10.1016/S0959-440X(01)00264-0. PMID 11751052.
- Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M (July 1992). "Active nuclear import of human immunodeficiency virus type 1 preintegration complexes". Proceedings of the National Academy of Sciences of the United States of America. 89 (14): 6580–4. Bibcode:1992PNAS...89.6580B. doi:10.1073/pnas.89.14.6580. PMC 49545. PMID 1631159.
- Bukrinsky MI, Haggerty S, Dempsey MP, Sharova N, Adzhubel A, Spitz L, et al. (October 1993). "A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells". Nature. 365 (6447): 666–9. Bibcode:1993Natur.365..666B. doi:10.1038/365666a0. PMC 9524217. PMID 8105392. S2CID 25678.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Bonaldo MF, Lennon G, Soares MB (September 1996). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Research. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Gallay P, Hope T, Chin D, Trono D (September 1997). "HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway". Proceedings of the National Academy of Sciences of the United States of America. 94 (18): 9825–30. Bibcode:1997PNAS...94.9825G. doi:10.1073/pnas.94.18.9825. PMC 23276. PMID 9275210.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Henderson BR, Percipalle P (December 1997). "Interactions between HIV Rev and nuclear import and export factors: the Rev nuclear localisation signal mediates specific binding to human importin-beta". Journal of Molecular Biology. 274 (5): 693–707. doi:10.1006/jmbi.1997.1420. PMID 9405152.
- Efthymiadis A, Briggs LJ, Jans DA (January 1998). "The HIV-1 Tat nuclear localization sequence confers novel nuclear import properties". The Journal of Biological Chemistry. 273 (3): 1623–8. doi:10.1074/jbc.273.3.1623. PMID 9430704.
- Vodicka MA, Koepp DM, Silver PA, Emerman M (January 1998). "HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection". Genes & Development. 12 (2): 175–85. doi:10.1101/gad.12.2.175. PMC 316441. PMID 9436978.
- Popov S, Rexach M, Zybarth G, Reiling N, Lee MA, Ratner L, et al. (February 1998). "Viral protein R regulates nuclear import of the HIV-1 pre-integration complex". The EMBO Journal. 17 (4): 909–17. doi:10.1093/emboj/17.4.909. PMC 1170440. PMID 9463369.
- Popov S, Rexach M, Ratner L, Blobel G, Bukrinsky M (May 1998). "Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex". The Journal of Biological Chemistry. 273 (21): 13347–52. doi:10.1074/jbc.273.21.13347. PMID 9582382.
- Fouchier RA, Meyer BE, Simon JH, Fischer U, Albright AV, González-Scarano F, Malim MH (July 1998). "Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex". Journal of Virology. 72 (7): 6004–13. doi:10.1128/JVI.72.7.6004-6013.1998. PMC 110405. PMID 9621063.