 | |
| Names | |
|---|---|
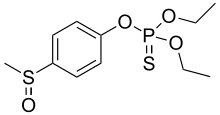
| Preferred IUPAC name
O,O-Diethyl O-[4-(methanesulfinyl)phenyl] phosphorothioate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.741 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H17O4PS2 | |
| Molar mass | 308.35 g·mol−1 |
| Appearance | Brown liquid or yellow oil[1] |
| Density | 1.20 g/mL (20°C)[1] |
| 0.2% (25°C) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
combustible[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[1] |
REL (Recommended) |
TWA 0.1 mg/m3[1] |
IDLH (Immediate danger) |
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Fensulfothion is an insecticide and nematicide. It is highly toxic and listed as an extremely hazardous substance.[2] It is widely used on corn, onions, rutabagas, pineapple, bananas, sugar cane, sugar beets, pea nuts, etc.[3]
External links
- Fensulfothion in the Pesticide Properties DataBase (PPDB)
References
- 1 2 3 4 5 6 NIOSH Pocket Guide to Chemical Hazards. "#0284". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Appendix A List of Extremely Hazardous Chemicals
- ↑ Sunil Paul, MM; Aravind, UK; Pramod, G; Aravindakumar, CT (April 2013). "Oxidative degradation of fensulfothion by hydroxyl radical in aqueous medium". Chemosphere. 91 (3): 295–301. Bibcode:2013Chmsp..91..295S. doi:10.1016/j.chemosphere.2012.11.033. PMID 23273737.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.