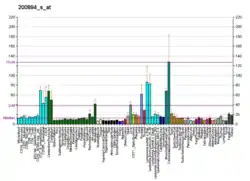
FK506-binding protein 4 is a protein that in humans is encoded by the FKBP4 gene.[5][6]
Function
The protein encoded by this gene is a member of the immunophilin protein family, which play a role in immunoregulation and basic cellular processes involving protein folding and trafficking. This encoded protein is a cis-trans prolyl isomerase that binds to the immunosuppressants FK506 and rapamycin. It has high structural and functional similarity to FK506-binding protein 1A (FKBP1A), but unlike FKBP1A, this protein does not have immunosuppressant activity when complexed with FK506. It interacts with interferon regulatory factor-4 and plays an important role in immunoregulatory gene expression in B and T lymphocytes. This encoded protein is known to associate with phytanoyl-CoA alpha-hydroxylase. It can also associate with two heat shock proteins (hsp90 and hsp70) and thus may play a role in the intracellular trafficking of hetero-oligomeric forms of the steroid hormone receptors. This protein correlates strongly with adeno-associated virus type 2 vectors (AAV) resulting in a significant increase in AAV-mediated transgene expression in human cell lines. Thus this encoded protein is thought to have important implications for the optimal use of AAV vectors in human gene therapy.[6]
Structure
This protein contains TPR repeats and has a PPlase domain.
Clinical significance
Recent research suggests that FKBP4 may play a role in preventing the Tau protein from turning pathogenic. This may prove significant for the development of new Alzheimer's drugs and for detecting the disease before the onset of clinical symptoms.[7]
Interactions
See also
- Glucocorticoid receptor
- Immunophilins
- FKBP5
- FKBP3 - A DNA binding FKBP. [10]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000004478 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000030357 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Peattie DA, Harding MW, Fleming MA, DeCenzo MT, Lippke JA, Livingston DJ, Benasutti M (November 1992). "Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes". Proceedings of the National Academy of Sciences of the United States of America. 89 (22): 10974–8. Bibcode:1992PNAS...8910974P. doi:10.1073/pnas.89.22.10974. PMC 50465. PMID 1279700.
- 1 2 "Entrez Gene: FKBP4 FK506 binding protein 4, 59kDa".
- ↑ Blair LJ, Baker JD, Sabbagh JJ, Dickey CA (April 2015). "The emerging role of peptidyl-prolyl isomerase chaperones in tau oligomerization, amyloid processing, and Alzheimer's disease". Journal of Neurochemistry. 133 (1): 1–13. doi:10.1111/jnc.13033. PMC 4361273. PMID 25628064.
- ↑ Chambraud B, Radanyi C, Camonis JH, Shazand K, Rajkowski K, Baulieu EE (December 1996). "FAP48, a new protein that forms specific complexes with both immunophilins FKBP59 and FKBP12. Prevention by the immunosuppressant drugs FK506 and rapamycin". The Journal of Biological Chemistry. 271 (51): 32923–9. doi:10.1074/jbc.271.51.32923. PMID 8955134.
- ↑ Neye H (March 2001). "Mutation of FKBP associated protein 48 (FAP48) at proline 219 disrupts the interaction with FKBP12 and FKBP52". Regulatory Peptides. 97 (2–3): 147–52. doi:10.1016/S0167-0115(00)00206-8. PMID 11164950. S2CID 20617551.
- ↑ Prakash, Ajit; Shin, Joon; Rajan, Sreekanth; Yoon, Ho Sup (2016-04-07). "Structural basis of nucleic acid recognition by FK506-binding protein 25 (FKBP25), a nuclear immunophilin". Nucleic Acids Research. 44 (6): 2909–2925. doi:10.1093/nar/gkw001. ISSN 0305-1048. PMC 4824100. PMID 26762975.
Further reading
- Schiene-Fischer C, Yu C (April 2001). "Receptor accessory folding helper enzymes: the functional role of peptidyl prolyl cis/trans isomerases". FEBS Letters. 495 (1–2): 1–6. doi:10.1016/S0014-5793(01)02326-2. PMID 11322937. S2CID 42263861.
- Yem AW, Tomasselli AG, Heinrikson RL, Zurcher-Neely H, Ruff VA, Johnson RA, Deibel MR (February 1992). "The Hsp56 component of steroid receptor complexes binds to immobilized FK506 and shows homology to FKBP-12 and FKBP-13". The Journal of Biological Chemistry. 267 (5): 2868–71. doi:10.1016/S0021-9258(19)50664-0. PMID 1371107.
- Tai PK, Albers MW, Chang H, Faber LE, Schreiber SL (May 1992). "Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex". Science. 256 (5061): 1315–8. Bibcode:1992Sci...256.1315T. doi:10.1126/science.1376003. PMID 1376003.
- Wiederrecht G, Hung S, Chan HK, Marcy A, Martin M, Calaycay J, Boulton D, Sigal N, Kincaid RL, Siekierka JJ (October 1992). "Characterization of high molecular weight FK-506 binding activities reveals a novel FK-506-binding protein as well as a protein complex". The Journal of Biological Chemistry. 267 (30): 21753–60. doi:10.1016/S0021-9258(19)36676-1. PMID 1383226.
- Sanchez ER, Faber LE, Henzel WJ, Pratt WB (May 1990). "The 56-59-kilodalton protein identified in untransformed steroid receptor complexes is a unique protein that exists in cytosol in a complex with both the 70- and 90-kilodalton heat shock proteins". Biochemistry. 29 (21): 5145–52. doi:10.1021/bi00473a021. PMID 2378870.
- Alnemri ES, Fernandes-Alnemri T, Nelki DS, Dudley K, DuBois GC, Litwack G (July 1993). "Overexpression, characterization, and purification of a recombinant mouse immunophilin FKBP-52 and identification of an associated phosphoprotein". Proceedings of the National Academy of Sciences of the United States of America. 90 (14): 6839–43. Bibcode:1993PNAS...90.6839A. doi:10.1073/pnas.90.14.6839. PMC 47028. PMID 8341706.
- Chambraud B, Radanyi C, Camonis JH, Shazand K, Rajkowski K, Baulieu EE (December 1996). "FAP48, a new protein that forms specific complexes with both immunophilins FKBP59 and FKBP12. Prevention by the immunosuppressant drugs FK506 and rapamycin". The Journal of Biological Chemistry. 271 (51): 32923–9. doi:10.1074/jbc.271.51.32923. PMID 8955134.
- Bruner KL, Derfoul A, Robertson NM, Guerriero G, Fernandes-Alnemri T, Alnemri ES, Litwack G (1998). "The unliganded mineralocorticoid receptor is associated with heat shock proteins 70 and 90 and the immunophilin FKBP-52". Receptors & Signal Transduction. 7 (2): 85–98. PMID 9392437.
- Miyata Y, Chambraud B, Radanyi C, Leclerc J, Lebeau MC, Renoir JM, Shirai R, Catelli MG, Yahara I, Baulieu EE (December 1997). "Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II: regulation of HSP90-binding activity of FKBP52". Proceedings of the National Academy of Sciences of the United States of America. 94 (26): 14500–5. Bibcode:1997PNAS...9414500M. doi:10.1073/pnas.94.26.14500. PMC 25035. PMID 9405642.
- Bermingham NA, Rauf S, Katsanis N, Martin JE, Hunter AJ, Fisher EM (March 1998). "The immunophilin FKBP4 (FKBP52/FKBP59) maps to the distal short arm of human chromosome 12". Mammalian Genome. 9 (3): 268. doi:10.1007/s003359900746. PMID 9501323. S2CID 8388657.
- Chambraud B, Radanyi C, Camonis JH, Rajkowski K, Schumacher M, Baulieu EE (March 1999). "Immunophilins, Refsum disease, and lupus nephritis: the peroxisomal enzyme phytanoyl-COA alpha-hydroxylase is a new FKBP-associated protein". Proceedings of the National Academy of Sciences of the United States of America. 96 (5): 2104–9. Bibcode:1999PNAS...96.2104C. doi:10.1073/pnas.96.5.2104. PMC 26744. PMID 10051602.
- Mamane Y, Sharma S, Petropoulos L, Lin R, Hiscott J (February 2000). "Posttranslational regulation of IRF-4 activity by the immunophilin FKBP52". Immunity. 12 (2): 129–40. doi:10.1016/S1074-7613(00)80166-1. PMID 10714679.
- Neye H (March 2001). "Mutation of FKBP associated protein 48 (FAP48) at proline 219 disrupts the interaction with FKBP12 and FKBP52". Regulatory Peptides. 97 (2–3): 147–52. doi:10.1016/S0167-0115(00)00206-8. PMID 11164950. S2CID 20617551.
- Galigniana MD, Radanyi C, Renoir JM, Housley PR, Pratt WB (May 2001). "Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus". The Journal of Biological Chemistry. 276 (18): 14884–9. doi:10.1074/jbc.M010809200. PMID 11278753.
- Qing K, Hansen J, Weigel-Kelley KA, Tan M, Zhou S, Srivastava A (October 2001). "Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression". Journal of Virology. 75 (19): 8968–76. doi:10.1128/JVI.75.19.8968-8976.2001. PMC 114465. PMID 11533160.
- Guo Y, Guettouche T, Fenna M, Boellmann F, Pratt WB, Toft DO, Smith DF, Voellmy R (December 2001). "Evidence for a mechanism of repression of heat shock factor 1 transcriptional activity by a multichaperone complex". The Journal of Biological Chemistry. 276 (49): 45791–9. doi:10.1074/jbc.M105931200. PMID 11583998.
- Davies TH, Ning YM, Sánchez ER (February 2002). "A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins". The Journal of Biological Chemistry. 277 (7): 4597–600. doi:10.1074/jbc.C100531200. PMID 11751894.
External links
- FKBP52 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)