| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3-Dioxolan-2-one | |||
| Other names
ethylene glycol carbonate[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.283 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H4O3 | |||
| Molar mass | 88.062 g·mol−1 | ||
| Appearance | White to yellow solid | ||
| Density | 1.3210 g/cm3 | ||
| Melting point | 34 to 37 °C (93 to 99 °F; 307 to 310 K) | ||
| Boiling point | 243.0 °C (469.4 °F; 516.1 K) | ||
| Soluble | |||
| Hazards[2] | |||
| GHS labelling: | |||
 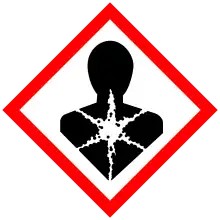 | |||
| Warning | |||
| H302, H319, H373 | |||
| P260, P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P310, P312, P314, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
| Flash point | 150 °C (302 °F; 423 K) | ||
| 465 °C (869 °F; 738 K) | |||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Ethylene carbonate (sometimes abbreviated EC) is the organic compound with the formula (CH2O)2CO. It is classified as the cyclic carbonate ester of ethylene glycol and carbonic acid. At room temperature (25 °C) ethylene carbonate is a transparent crystalline solid, practically odorless and colorless, and somewhat soluble in water. In the liquid state (m.p. 34-37 °C) it is a colorless odorless liquid.[3]
Production and reactions
Ethylene carbonate is produced by the reaction between ethylene oxide and carbon dioxide. The reaction is catalyzed by a variety of cations and complexes:[4][5]
- (CH2)2O + CO2 → (CH2O)2CO
In the laboratory, ethylene carbonate can also be produced from the reaction of urea and ethylene glycol using zinc oxide as a catalyst at a temperature of 150 °C and a pressure of 3 kPa:[6]
- (NH2)2CO + HO−CH2CH2−OH → (CH2O)2CO + 2 NH3
Ethylene carbonate (and propylene carbonate) may be converted to dimethyl carbonate (a useful solvent and a mild methylating agent) via transesterification by methanol:
- C2H4CO3 + 2 CH3OH → CH3OCO2CH3 + HOC2H4OH
The transesterfication of ethylene carbonate by methanol can be catalyzed by a high surface area (thermally exfoliated) graphitic carbon nitride (g-C3N4) materials. This method reduces the chance of metal or halide contamination, and can offer yields of up to 60% at a temperature of 393 K.[7]
Dimethyl carbonate may itself be similarly transesterified to diphenyl carbonate, a phosgene-substitute:[4]
- CH3OCO2CH3 + 2 PhOH → PhOCO2Ph + 2 MeOH
Applications
Ethylene carbonate is used as a polar solvent with a molecular dipole moment of 4.9 D,[8][9] only 0.1 D lower than that of propylene carbonate.
It can be used as a high permittivity component of electrolytes in lithium batteries and lithium-ion batteries. Other components like diethyl carbonate, ethyl methyl carbonate, dimethyl carbonate and methyl acetate can be added to those electrolytes in order to decrease the viscosity and melting point.[10]
Ethylene carbonate was a universal component of an electrolyte in earlier (prior to ca. 2010) lithium-ion batteries, since it is responsible for the formation of the solid electrolyte interphase on the anode. Since EC is solid at room temperature, it was mixed with propylenecarbonate. As dimethylcarbonate and other dialkylcarbonates became commercially available, they replaced ethylene carbonate in some modern lithium-ion batteries.
A typical sodium intercalation type battery would use an electrolyte consisting of: fluoroethylene carbonate (FEC) (99%), metallic Na (99.9%), and 1.0 M sodium perchlorate (NaClO4) solutions in ethylene carbonate and diethyl carbonate (EC/DEC), 1:1 v/v% battery-grade, mixed with FEC (10% by weight).[11]
Ethylene carbonate is also used as plasticizer, and as a precursor to vinylene carbonate, which is used in polymers and in organic synthesis.
Oxalyl chloride is produced commercially from ethylene carbonate. Photochlorination gives the tetrachloroethylene carbonate:[12]
- C2H4O2CO + 4 Cl2 → C2Cl4O2CO + 4 HCl
The tetrachloride is degraded to oxalyl chloride by amine catalysts.
- C2Cl4O2CO → C2O2Cl2 + COCl2
See also
External links
References
- ↑ "CID 7303 -- PubChem Compound Summary". pubchem.ncbi.nlm.nih.gov. Retrieved 2008-03-15.
- ↑ "C&L Inventory". echa.europa.eu.
- ↑ JEFFSOL ETHYLENE CARBONATE Archived 2012-07-22 at the Wayback Machine catalog entry at www.huntsman.com. Accessed on 2010-02-18.
- 1 2 Buysch, Hans-Josef (2012). "Carbonic Esters". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_197. ISBN 978-3527306732.
- ↑ Comerford, James W.; Ingram, Ian D. V.; North, Michael; Wu, Xiao (2015). "Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings". Green Chemistry. 17 (4): 1966–1987. doi:10.1039/C4GC01719F. S2CID 96255105.
- ↑ Bhalchandra M. Bhanage; Shin-ichiro Fujita (2003). "Transesterification of urea and ethylene glycol to ethylene carbonate as an important step for urea based dimethyl carbonate synthesis". Green Chemistry. 5 (4): 429–432. doi:10.1039/b304182d. S2CID 97286880.
- ↑ Gan, Yu-Lin; Hu, Xiao-Qian; Wen, Lin-Zhi; Xu, Jie; Xue, Bing (2020-02-24). "Metal-free synthesis of dimethyl carbonate via transesterification of ethylene carbonate catalyzed by graphitic carbon nitride materials". New Journal of Chemistry. 44 (8): 3215–3223. doi:10.1039/C9NJ04530A. ISSN 1369-9261. S2CID 213404364.
- ↑ Ralph P. Seward; Ernest C. Vieira (1958). "The Dielectric Constants of Ethylene Carbonate and of Solutions of Ethylene Carbonate in Water, Methanol, Benzene and Propylene Carbonate". J. Phys. Chem. 62 (1): 127–128. doi:10.1021/j150559a041.
- ↑ Richard Payne; Ignatius E. Theodorou (1972). "Dielectric properties and relaxation in ethylene carbonate and propylene carbonate". J. Phys. Chem. 76 (20): 2892–2900. doi:10.1021/j100664a019.
- ↑ E. R. Logan; J. R. Dahn (2018). "A Study of the Physical Properties of Li-Ion Battery Electrolytes Containing Esters". J. Electrochem. Soc. 165 (2): A21–A30. doi:10.1149/2.0271802jes. OSTI 1469344.
- ↑ Youssef Sayed, Sayed; Kalisvaart, W. Peter; Olsen, Brian; Luber, Erik; Buriak, Jillian (2020-07-13). "Stabilizing Tin Anodes in Sodium-Ion Batteries by Alloying with Silicon". figshare. doi:10.26434/chemrxiv.12642956.v1. S2CID 243291502. Retrieved 2021-02-24.
- ↑ Pfoertner, Karl-Heinz (2000). "Photochemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_573. ISBN 978-3527306732.