 | |
| Clinical data | |
|---|---|
| Other names | Estradiol 3-propanoate; 3-Propanoylestradiol; 3,17β-Hydroxyestra-1,3,5(10)-trien-3-yl propionate |
| Routes of administration | Intramuscular injection, vaginal[1] |
| Drug class | Estrogen; Estrogen ester |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H28O3 |
| Molar mass | 328.452 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
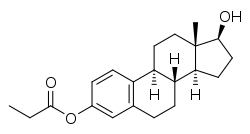
Estradiol 3-propionate, or 3-propanoylestradiol, also known as estra-1,3,5(10)-triene-3,17β-diol 3-propionate, is a semisynthetic, steroidal estrogen that was never marketed.[1][2] It is an estrogen ester, specifically, a propionic acid ester of estradiol, and acts as a prodrug to it in vivo.[1][2] The chemical structure of estradiol 3-propionate is contained within estradiol dipropionate, estrapronicate, and orestrate,[3] all of which are also estradiol esters.[2]
See also
References
- 1 2 3 Woolfson AD, Elliott GR, Gilligan CA, Passmore CM (1999). "Design of an intravaginal ring for the controlled delivery of 17 beta-estradiol as its 3-acetate ester". J Control Release. 61 (3): 319–28. doi:10.1016/s0168-3659(99)00148-0. PMID 10477804.
- 1 2 3 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 898. ISBN 978-1-4757-2085-3.
- ↑ PubChem. "Estradiol". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-11-02.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.