 | |
| Names | |
|---|---|
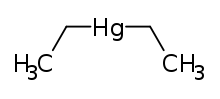
| IUPAC name
diethylmercury | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.001 |
| EC Number |
|
| MeSH | C007378 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C 4H 10Hg (C 2H 5) 2Hg | |
| Molar mass | 258.71 g/mol |
| Appearance | Colorless liquid |
| Odor | Sweet |
| Density | 2.446 g/ml |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 156 to 157 °C (313 to 315 °F; 429 to 430 K) |
| Insoluble | |
| Solubility | Ethers, hydrocarbons, THF |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Flammable, extremely toxic |
| GHS labelling: | |
   | |
| Danger | |
| H225, H300+H310+H330, H373, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P302+P350, P304+P340, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 | |
| Flash point | N/A |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Diethylmercury is a flammable, colorless liquid, and one of the strongest known neurotoxins. This organomercury compound is described as having a slightly sweet smell, though inhaling enough fumes to notice this would be hazardous.[1] This chemical can cross the blood–brain barrier, causing permanent brain damage. It is, however, considerably less toxic than dimethylmercury.
Synthesis
Diethylmercury can be obtained from the reaction between ethylmagnesium bromide and mercury(II) chloride.[2]
- 2 C2H5MgBr + HgCl2 → Hg(C2H5)2 + MgBr2 + MgCl2
Other methods are also known.[3]
See also
- Dimethylmercury, a related compound
- Ethylmercury
- Mercury poisoning
References
- ↑ "Diethyl Mercury | 627-44-1". Archived from the original on 2018-10-27. Retrieved 2009-01-28.
- ↑ Brauer, Georg. Handbuch der präparativen anorganischen Chemie Bd. 2. Baudler, Marianne (3rd ed.). Stuttgart. p. 1063. ISBN 978-3-432-87813-3. OCLC 310719490.
- ↑ Kolbe, Hermann (1860). Ausführliches Lehrbuch der organischen Chemie, Volume 2. p. 964.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.