Defensin, alpha 5 (DEFA5) also known as human alpha defensin 5 (HD5) is a protein that in humans is encoded by the DEFA5 gene.[3][4] DEFA5 is expressed in the Paneth cells of the ileum.[5]
Defensins are a family of microbicidal and cytotoxic peptides (antimicrobial peptides; AMP) that are involved in host defense, and help to maintain homeostasis of intestinal microbiota. DEFA5 is the main AMP that controls the enteric microbiota composition by selective killing of bacterial pathogens while preserving commensals.[6]
Structure
Defensins are small cationic peptides linked via three intra-molecular disulfide bridges, and contain six intra-molecular cysteine residues which form an unalterable and specific pattern of disulfide bridges which protects them from proteolysis and maintains function in the intestinal lumen.[7][8] Members of the defensin family are highly similar in protein sequence and distinguished by a conserved cysteine motif.
Gene and tissue distribution
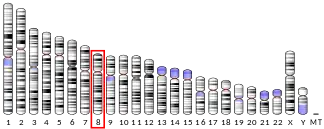
Several of the human alpha defensin genes appear to be clustered on chromosome 8. The protein encoded by this gene, α-defensin-5, is highly expressed in the secretory granules of Paneth cells of the ileum.[3]
Function
In addition to antimicrobial activity, inactivation and neutralization of several bacterial toxins, especially an inhibitory potency against Clostridioides difficile toxins were reported.[9][10] α-defensin-5 is able to inhibit all three C. difficile toxins A (TcdA), B (TcdB) and CDT in the concentration-dependent manner, and inhibitory mechanism is different for each of them. TcdA and TcdB are inhibited by co-precipitation with DEFA5, and CDT is inhibited by the inactivation of the CDTb pore.[11] In addition to toxin neutralization, DEFA5 is capable to direcrly kill C. difficile cells by damaging the bacterial wall[12]
References
- 1 2 3 ENSG00000285251 GRCh38: Ensembl release 89: ENSG00000164816, ENSG00000285251 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- 1 2 "Entrez Gene: DEFA5 defensin, alpha 5, Paneth cell-specific".
- ↑ Jones DE, Bevins CL (January 1993). "Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel". FEBS Letters. 315 (2): 187–92. doi:10.1016/0014-5793(93)81160-2. PMID 8417977. S2CID 45099115.
- ↑ Zhao C, Wang I, Lehrer RI (November 1996). "Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells". FEBS Letters. 396 (2–3): 319–22. doi:10.1016/0014-5793(96)01123-4. PMID 8915011. S2CID 22367037.
- ↑ Bevins CL, Salzman NH (May 2011). "Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis". Nature Reviews. Microbiology. 9 (5): 356–68. doi:10.1038/nrmicro2546. PMID 21423246. S2CID 39332656.
- ↑ Ganz T, Lehrer RI (August 1994). "Defensins". Current Opinion in Immunology. 6 (4): 584–9. doi:10.1016/0952-7915(94)90145-7. PMID 7946046.
- ↑ Selsted ME, Harwig SS (March 1989). "Determination of the disulfide array in the human defensin HNP-2. A covalently cyclized peptide". The Journal of Biological Chemistry. 264 (7): 4003–7. doi:10.1016/S0021-9258(19)84952-9. PMID 2917986.
- ↑ Kim C, Slavinskaya Z, Merrill AR, Kaufmann SH (October 2006). "Human alpha-defensins neutralize toxins of the mono-ADP-ribosyltransferase family". The Biochemical Journal. 399 (2): 225–9. doi:10.1042/BJ20060425. PMC 1609915. PMID 16817779.
- ↑ Giesemann T, Guttenberg G, Aktories K (June 2008). "Human alpha-defensins inhibit Clostridium difficile toxin B". Gastroenterology. 134 (7): 2049–58. doi:10.1053/j.gastro.2008.03.008. PMID 18435932.
- ↑ Korbmacher M, Fischer S, Landenberger M, Papatheodorou P, Aktories K, Barth H (2020). "Human α-Defensin-5 Efficiently Neutralizes Clostridioides difficile Toxins TcdA, TcdB, and CDT". Frontiers in Pharmacology. 11: 1204. doi:10.3389/fphar.2020.01204. PMC 7435013. PMID 32903430.
- ↑ Furci L, Baldan R, Bianchini V, Trovato A, Ossi C, Cichero P, Cirillo DM (March 2015). Morrison RP (ed.). "New role for human α-defensin 5 in the fight against hypervirulent Clostridium difficile strains". Infection and Immunity. 83 (3): 986–95. doi:10.1128/IAI.02955-14. PMC 4333456. PMID 25547793.
Further reading
- Mallow EB, Harris A, Salzman N, Russell JP, DeBerardinis RJ, Ruchelli E, Bevins CL (February 1996). "Human enteric defensins. Gene structure and developmental expression". The Journal of Biological Chemistry. 271 (8): 4038–45. doi:10.1074/jbc.271.8.4038. PMID 8626737.
- Bevins CL, Jones DE, Dutra A, Schaffzin J, Muenke M (January 1996). "Human enteric defensin genes: chromosomal map position and a model for possible evolutionary relationships". Genomics. 31 (1): 95–106. doi:10.1006/geno.1996.0014. PMID 8808285.
- Porter EM, Liu L, Oren A, Anton PA, Ganz T (June 1997). "Localization of human intestinal defensin 5 in Paneth cell granules". Infection and Immunity. 65 (6): 2389–95. doi:10.1128/IAI.65.6.2389-2395.1997. PMC 175331. PMID 9169779.
- Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC (May 1998). "Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract". The American Journal of Pathology. 152 (5): 1247–58. PMC 1858596. PMID 9588893.
- Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, et al. (June 2002). "Paneth cell trypsin is the processing enzyme for human defensin-5". Nature Immunology. 3 (6): 583–90. doi:10.1038/ni797. PMID 12021776. S2CID 22022089.
- Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL (April 2003). "Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin". Nature. 422 (6931): 522–6. Bibcode:2003Natur.422..522S. doi:10.1038/nature01520. PMID 12660734. S2CID 1096692.
- Szyk A, Wu Z, Tucker K, Yang D, Lu W, Lubkowski J (December 2006). "Crystal structures of human alpha-defensins HNP4, HD5, and HD6". Protein Science. 15 (12): 2749–60. doi:10.1110/ps.062336606. PMC 2242434. PMID 17088326.
- Wu Z, Ericksen B, Tucker K, Lubkowski J, Lu W (September 2004). "Synthesis and characterization of human alpha-defensins 4-6". The Journal of Peptide Research. 64 (3): 118–25. doi:10.1111/j.1399-3011.2004.00179.x. PMID 15317502.
- de Leeuw E, Burks SR, Li X, Kao JP, Lu W (February 2007). "Structure-dependent functional properties of human defensin 5". FEBS Letters. 581 (3): 515–20. doi:10.1016/j.febslet.2006.12.036. PMC 1832120. PMID 17250830.