 | |
| Names | |
|---|---|
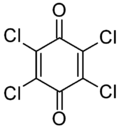
| IUPAC name
2,3,5,6-Tetrachlorocyclohexa-2,5-diene-1,4-dione | |
| Other names
p-Chloranil; Tetrachloro-1,4-benzoquinone; Tetrachloro-p-benzoquinone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.887 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6Cl4O2 | |
| Molar mass | 245.86 g·mol−1 |
| Appearance | Yellow solid |
| Melting point | 295 to 296 °C (563 to 565 °F; 568 to 569 K) |
| -112.6·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H315, H319, H410 | |
| P264, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule[2] that functions as a mild oxidant.
Synthesis and use as reagent
Chloranil is produced by chlorination of phenol to give hexachlorocyclohexa-2,5-dien-1-one ("hexachlorophenol"). Hydrolysis of the dichloromethylene group in this dienone gives chloranil:[3]
- C6H5OH + 6 Cl2 → C6Cl6O + 6 HCl
- C6Cl6O + H2O → C6Cl4O2 + 2 HCl
Chloroanil serves as a hydrogen acceptor. It is more electrophilic than quinone itself. It is used for the aromatization reactions, such as the conversion of cyclohexadienes to the benzene derivatives.[4]
Chloranil is used to test for free secondary amines. This test is useful for checking for the presence of proline derivatives. It is also a good test for the successful deprotection of a secondary amine. Secondary amines react with chloranil to give a brown/red/orange derivative, the colour depending on the amine. In these reactions, the amine displaces chloride from the ring of the quinone.
Commercial applications
It is a precursor to many dyes, such as pigment violet 23 and diaziquone (AZQ), a cancer chemotherapeutic agent.
See also
References
- ↑ Chloranil at Sigma-Aldrich
- ↑ J.-M. Lü, S. V. Rosokha, I. S. Neretin and J. K. Kochi, "Quinones as Electron Acceptors. X-Ray Structures, Spectral (EPR, UV-vis) Characteristics and Electron-Transfer Reactivities of Their Reduced Anion Radicals as Separated vs Contact Ion Pairs" Journal of the American Chemical Society 2006 128, 16708-16719.doi:10.1021/ja066471o
- ↑ François Muller and Liliane Caillard "Chlorophenols" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim, 2011. doi:10.1002/14356007.a07_001.pub2
- ↑ Derek R. Buckle "Chloranil" in Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley. doi:10.1002/047084289X.rc057
External links
- Chloranil in the Pesticide Properties DataBase (PPDB)