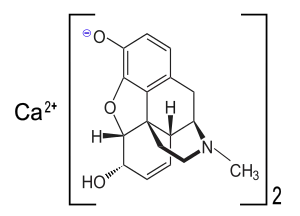
Chemical structure of a component of the calcium morphenate mixture
Calcium morphenate is a calcium salt of morphine which is produced by using calcium bases to raise the pH of an aqueous solution of opium alkaloids to around 9.0.[1] This was a method used in pharmaceutical manufacturing to separate morphine from other alkaloids and inert materials from the opium solution. Variations on this route are still used in Afghanistan.[2] When poppy straw concentrate or opium latex is dissolved in hot water and the calcium base is added, calcium morphenate is formed. Treatment with a weak acid such as ammonium chloride then causes morphine freebase to precipitate, leaving codeine and other alkaloids of the plant in solution.

Manufacture of heroin form morphine
References
- ↑ US 2062324, "Method of Extraction of Morphine and Related Derivatives"
- ↑ U. Zerell, B. Ahrens and P. Gerz (2005). "Documentation of a heroin manufacturing process in Afghanistan" (PDF). Bulletin on Narcotics. LVII (1 and 2).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.