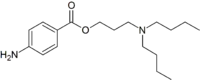 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-(Dibutylamino)propyl 4-aminobenzoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.214 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H30N2O2 | |
| Molar mass | 306.443 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Butacaine is a white crystalline ester used as a local anesthetic.
Synthesis
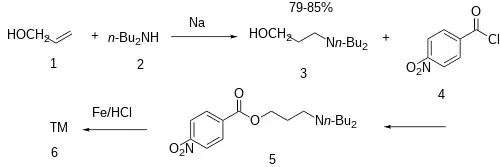
The addition of metallic sodium is added to a mixture of allyl alcohol (1) and N,N-dibutylamine (2) gives the conjugate addition product and hence 3-dibutylamino-1-propanol [2050-51-3] (3). Esterification of this intermediate with para-nitrobenzoyl chloride [122-04-3] (4) gives CID:4588466 (5). The reduction of the nitro group completed the synthesis of butacaine (6).
See also
References
- ↑ Burnett, W. B.; Jenkins, R. L.; Peet, C. H.; Dreger, E. E.; Adams, Roger (1937). "Dialkylaminoalkanol Esters of p-Aminobenzoic Acid". Journal of the American Chemical Society 59 (11): 2248–2252. doi:10.1021/ja01290a041.
- ↑ Kaye, Irving Allan; Roberts, I. Melville (1951). "Dialkylaminoalkyl Esters of 2-Amino-6-carboxybenzothiazole". Journal of the American Chemical Society 73 (10): 4762–4764. doi:10.1021/ja01154a084.
- ↑ Oliver Kamm, Roger Adams, Volwiler Ernest H., U.S. Patent 1,358,751 (1920 to Abbott Lab).
- ↑ Adams Roger, Ernest H Volwiler, U.S. Patent 1,676,470 (1928 to Abbott Lab).
- ↑ Weston Arthur W, U.S. Patent 2,437,984 (1948 to Abbott Lab).
- ↑ Anon., GB 191122 (1922-12-27 to Abbott Lab).
- ↑ Kurihara, Tozaburo; Niwa, Hiroshi; Chiba, Katsuichi (1954). "Synthesis of γ-Alkylaminopropanols". YAKUGAKU ZASSHI. 74 (7): 763–766. doi:10.1248/yakushi1947.74.7_763.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.