 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /bəˈnæzəprɪl/ |
| Trade names | Lotensin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692011 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 96.7% |
| Metabolism | Liver glucuronidation |
| Elimination half-life | 10-11 hours |
| Excretion | Kidney and bile duct |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
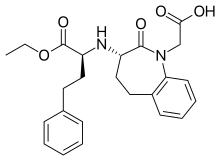
| Formula | C24H28N2O5 |
| Molar mass | 424.497 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Benazepril, sold under the brand name Lotensin among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease.[1] It is a reasonable initial treatment for high blood pressure.[1] It is taken by mouth.[1] Versions are available as the combinations benazepril/hydrochlorothiazide and benazepril/amlodipine.[1]
Benazepril was invented by: Dr. Jeffrey William Herbert Watthey for Ciba Geigy, now Novartis.
Common side effects include feeling tired, dizziness, cough, and light-headedness with standing.[1] Serious side effects may include kidney problems, low blood pressure, high blood potassium, and angioedema.[1] Use in pregnancy may harm the baby, while use when breastfeeding may be safe.[2] It is an ACE inhibitor and works by decreasing renin-angiotensin-aldosterone system activity.[1]
Benazepril was patented in 1981 and came into medical use in 1990. It was created by the chemist Mahesh Desai. [3] It is available as a generic medication.[1] In 2020, it was the 141st most commonly prescribed medication in the United States, with more than 4 million prescriptions.[4][5]
Structure activity relationship
Benazepril hydrochloride's OCH2CH3 group must be metabolized to form benazeprilat—the active form of the molecule—in order to inhibit the ACE enzyme. The bulky cyclic structure is resistant to hydrolysis. The nitrogen within the ring makes the bulky cyclic structure especially difficult to break down, and can account for the drug's pharmacokinetic profile, in which the duration of action is 24 hours.
Medical uses
It is useful for high blood pressure, heart failure, and diabetic kidney disease.[1] It is a reasonable initial treatment for high blood pressure.[1] Other reasonable initial options include angiotensin II receptor antagonists, calcium-channel blockers, and thiazide diuretics.[1]
Side effects
The most common side effects patients experience are a headache or a chronic cough. The chronic cough develops in about 20% of patients treated,[6] and those patients that experience it find it develops after a few months of use. Anaphylaxis, angioedema, and elevation of potassium levels are more serious side effects that can also occur.
Contraindications
Benazepril should be discontinued during pregnancy and in women planning to become pregnant, as it can harm the fetus.[7]
Dosage forms
It is also available in combination with hydrochlorothiazide, under the trade name Lotensin HCT, and with amlodipine (Lotrel).
Veterinary use
Under the brand names Fortekor (Novartis) and VetACE (Jurox Animal Health), benazepril is used to treat congestive heart failure in dogs[8][9] and chronic kidney failure in cats and dogs.[10]
References
- 1 2 3 4 5 6 7 8 9 10 11 "Benazepril Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ↑ "Benazepril Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 468. ISBN 9783527607495.
- ↑ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ↑ "Benazepril - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ↑ Dykewicz MS (April 2004). "Cough and Angioedema From Angiotensin-Converting Enzyme Inhibitors: New Insights Into Mechanisms and Management". Medscape. Retrieved 2 April 2014.
- ↑ "Lotensin package insert" (PDF). FDA. 2011. Retrieved 9 December 2020.
- ↑ King JN, Mauron C, Kaiser G (December 1995). "Pharmacokinetics of the active metabolite of benazepril, benazeprilat, and inhibition of plasma angiotensin-converting enzyme activity after single and repeated administrations to dogs". American Journal of Veterinary Research. 56 (12): 1620–1628. PMID 8599524.
- ↑ O'Grady MR, O'Sullivan ML, Minors SL, Horne R (2009). "Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman Pinschers". Journal of Veterinary Internal Medicine. 23 (5): 977–983. doi:10.1111/j.1939-1676.2009.0346.x. PMID 19572914.
- ↑ "Fortekor Flavor Tabs (5 mg) (Canada) for Animal Use". Drugs.com. Retrieved 9 December 2020.
External links
- "Benazepril". Drug Information Portal. U.S. National Library of Medicine.