 | |
| Names | |
|---|---|
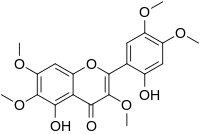
| IUPAC name
2′,5-Dihydroxy-3,4′,5′,6,7-pentamethoxyflavone | |
| Systematic IUPAC name
5-Hydroxy-2-(2-hydroxy-4,5-dimethoxyphenyl)-3,6,7-trimethoxy-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H20O9 | |
| Molar mass | 404.371 g·mol−1 |
| Density | 1.443 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Brickellin is an O-methylated flavonol.[1] It can be found in Brickellia veronicifolia.[2]
References
- ↑ Iinuma, M (1985). "Synthesis and revised structure of the flavone brickellin". Phytochemistry. 24 (6): 1367–1368. doi:10.1016/S0031-9422(00)81135-8.
- ↑ Brickellin, a novel flavone from Brickellia veronicaefolia and B. chlorolepis. Roberts M. F., Timmermann B. N., Mabry T. J., Brown R. and Matlin S. A., Phytochemistry, 1984, volume 23, no 1, pages 163-165, INIST 9471694
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.