 | |
| Names | |
|---|---|
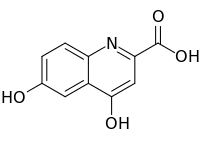
| Preferred IUPAC name
4,6-Dihydroxyquinoline-2-carboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H7NO4 | |
| Molar mass | 205.169 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
6-Hydroxykynurenic acid is a constituent of ginkgo and an amino acid.[1] It is a derivative of kynurenic acid and has similarly been found to antagonize AMPA and NMDA, as well as their corresponding receptors.[2]
See also
References
- ↑ van Beek, Teris A (2002-08-16). "Chemical analysis of Ginkgo biloba leaves and extracts". Journal of Chromatography A. Chromatographic and Electrophoretic Analysis of Secondary Metabolites of Plants. 967 (1): 21–55. doi:10.1016/S0021-9673(02)00172-3. ISSN 0021-9673. PMID 12219929.
- ↑ Weber, Marco; Dietrich, Dirk; Gräsel, Ines; Reuter, Gerhard; Seifert, Gerald; Steinhäuser, Christian (2001-12-20). "6-Hydroxykynurenic acid and kynurenic acid differently antagonise AMPA and NMDA receptors in hippocampal neurones: 6-Hydroxykynurenate blocks glutamate receptors". Journal of Neurochemistry. 77 (4): 1108–1115. doi:10.1046/j.1471-4159.2001.00340.x. PMID 11359876. S2CID 23178914.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.