 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 48 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
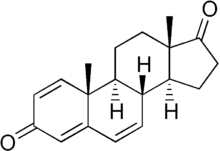
| Formula | C19H22O2 |
| Molar mass | 282 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
1,4,6-Androstatriene-3,17-dione (ATD) is a potent irreversible aromatase inhibitor that inhibits estrogen biosynthesis by permanently binding and inactivating aromatase in adipose and peripheral tissue.[1] It is used to control estrogen synthesis.[2]
ATD was present in some over-the-counter bodybuilding supplements until 2009, as well as Topical ATD solutions that work transdermally. The product was developed and commercialized in the dietary supplement market place by industry journeyman Bruce Kneller, who holds a United States Patent for use of the compound and related compounds (#7,939,517) and Gaspari Nutrition. ATD has many names in sports supplements including: 1,4,6 etiollochan-dione, 3, 17-keto-etiochol-triene, androst-1,4,6-triene-3,17-dione and many others. These all refer to CAS# 633-35-2.
ATD may cause a positive test for the anabolic steroid Boldenone, of which it is a possible metabolite and production contaminant. ATD is also prohibited in amateur and professional sports which forbids aromatase inhibitors.[3]
A related agent is exemestane (Aromasin).
References
- ↑ Covey DF, Hood WF (April 1981). "Enzyme-generated intermediates derived from 4-androstene-3,6,17-trione and 1,4,6-androstatriene-3,17-dione cause a time-dependent decrease in human placental aromatase activity". Endocrinology. 108 (4): 1597–1599. doi:10.1210/endo-108-4-1597. PMID 7472286.
- ↑ Adkins-Regan E, Leung CH (December 2006). "Sex steroids modulate changes in social and sexual preference during juvenile development in zebra finches". Hormones and Behavior. Elsevier Inc. 50 (5): 772–778. doi:10.1016/j.yhbeh.2006.07.003. PMID 16919276. S2CID 23869106.
- ↑ Parr MK, Fusshöller G, Schlörer N, Opfermann G, Piper T, Rodchenkov G, Schänzer W (January 2009). "Metabolism of androsta-1,4,6-triene-3,17-dione and detection by gas chromatography/mass spectrometry in doping control". Rapid Communications in Mass Spectrometry. 23 (2): 207–218. Bibcode:2009RCMS...23..207P. doi:10.1002/rcm.3861. PMID 19089863.
Further reading
- Ellinwood WE, Hess DL, Roselli CE, Spies HG, Resko JA (December 1984). "Inhibition of aromatization stimulates luteinizing hormone and testosterone secretion in adult male rhesus monkeys". The Journal of Clinical Endocrinology and Metabolism. 59 (6): 1088–1096. doi:10.1210/jcem-59-6-1088. PMID 6541658.