| Subject classification: this is a chemistry resource. |
| Type classification: this is a lesson resource. |
A harmonic oscillator (quantum or classical) is a particle in a potential energy well given by V(x)=½kx². k is called the force constant. It can be seen as the motion of a small mass attached to a string, or a particle oscillating in a well shaped as a parabola. This is a very important model because most potential energies can be approximated as parabolas near their minima, and the model allows us to understand the vibrations in molecular systems.
The classical harmonic oscillator
The classical harmonic oscillator is often studied in elementary physics. The solution of Newton's equation gives x(t) = A sin(ωt + φ) (Eq. 1) where is the angular frequency (Eq. 2), A is the amplitude of the motion and φ is its phase.
The total energy does not depend on time.
(Eq. 3)
Etot is proportional to the square of the amplitude and can be any positive number.
The quantum harmonic oscillator
To find the eigenvalues and eigenfunction of the quantum harmonic oscillator you should solve the following problem: (Eq. 4)
In this case, the solution will be given without proof. The eigenvalues of the harmonic oscillator are: Ev = ħω(½ + v) where v=0,1,2,... (Eq. 5)
The vibrational quantum number is indicated by v and can take any integer starting from zero. ω is the same angular frequency used for the classical oscillator.
To express the eigenfunctions in a simpler form it is convenient to divide Equation 4 by ħω which gives (Eq. 6)
And by defining a new adimensional variable , the equation takes a very simple form (the same for all oscillators): (Eq. 7)
This is just done to give the eigenfunctions a simpler form. The first four eigenfunctions of the harmonic oscillator Hamiltonian are:
- (Eq. 8)
N are just normalization constants. The general structure of the harmonic oscillator wavefunction is: (Eq. 9)
In other words, they are the product of a Gaussian function and a polynomial Hv(y) (known by mathematicians as Hermite polynomials and are given in tables for consultation).
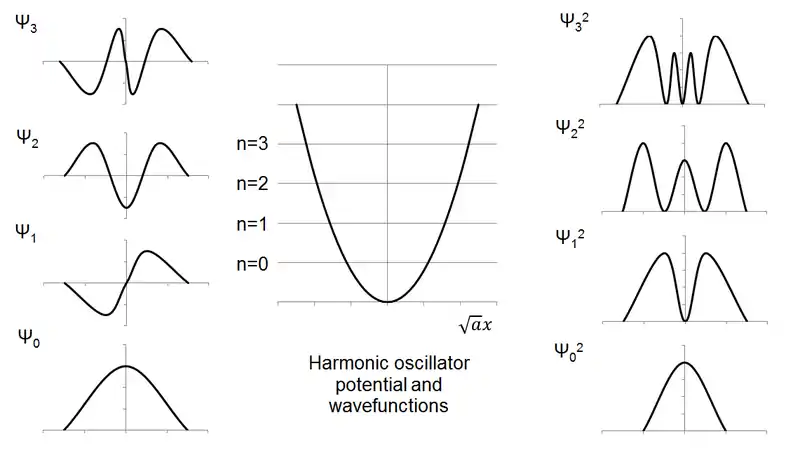
Note that, as in the case of the particle in the box, the number of nodes increases with increasing quantum number and energy.
However, unlike the classical harmonic oscillator, not only are there discrete levels but the ground state does not have zero energy, rather a finite amount (½ħω). This minimal amount of energy is known as the zero point energy.
Tunnelling in classically forbidden region
From the wavefunction ψv(x) you can calculate the probability of finding a particle at a given point by taking the modulus squared |ψv(x)|². Consider for example the normalized probability density for the ground state. A classical oscillator with the same energy Ev=0=½ħω would oscillate between two turning points -xT and +xT where . But the quantum oscillator has some probability of being found at x<-xT and x>xT in the classically forbidden region. A particle can be in the classically forbidden region only if it is allowed to have negative kinetic energy, which is impossible in classical mechanics. The phenomenon of quantum particles being able to be in regions of space forbidden to the classical particle is called quantum mechanical tunnelling.
The correspondence principle
We have been listing phenomena (tunnelling, nodal plane, discrete levels of energy) which make quantum mechanic behaviour very different than classical behaviour. Why does classical physics seem to work fine for macroscopic objects? The answer is complicated but a good starting point is the correspondence principle: at very high quantum numbers and energies, the quantum mechanical behaviour coincides with the classical behaviour.
When we observe a macroscopic oscillator, it could be described quantum mechanically but the quantum number would be enormous (see Exercise). If the correspondence principle is correct the quantum and classical probability of finding a particle in a particular position should approach each other for very high energies. This is what happens (question 10).
|
Exercise
|
Next: Lesson 5 - Further Particles in the Box and Polar Coordinates