- See also Biochemistry
- Carbon Chemistry
- Organic chemistry is the study of all compounds that contain bonds between Carbon atoms.
- Four major elements that are found in biological organic compounds are:
- Carbon, Oxygen, Hydrogen, Nitrogen, Sulfur, and Phosphorus
Carbon
- Lewis Dot Structure and the Structual Formula for a carbon atom
 Lewis Dot Structure for a carbon atom
Lewis Dot Structure for a carbon atom
How many bonds can carbon make with other atoms? FOUR
Importance of Carbon

Methane
- Carbon can make 4 covalent bonds with other atoms. This makes it flexible; it can bond with many elements.
- A carbon atom can bond with another carbon atom to create long carbon chains/carbon ring structures.
Carbon atoms bonded to hydrogen atoms are known as Hydrocarbons, an example is Methane.
Macromolecules

Monomers and Polymers Puzzle comparison
- What is a macromolecule?
- A giant molecule made from 100 to 1,000 of smaller molecules.
- What are macromolecules made up of?
- Monomers
- What is polymerization?
- When monomer ions join together to form polymers
- What is dehydration synthesis?
- When a water molecule is removed to join 2 monomers together.
- What is hydrolysis?
- When a water molecule is split to break bonds between monomers.
Monomer for each Macromolecule
 Carbohydrate Monomer = Monosaccharides (monomers)
Carbohydrate Monomer = Monosaccharides (monomers) Lipid Monomer = Triglycerides (monomers)
Lipid Monomer = Triglycerides (monomers) Protein Monomer = Amino Acids (monomers)
Protein Monomer = Amino Acids (monomers) Nucleic Acid Monomer = Nucleotides (monomers)
Nucleic Acid Monomer = Nucleotides (monomers)
Four major macromolecules

- What are the four major macromolecules in living things?
| Macromolecule | Example |
|---|---|
| Carbohydrates | Sugar |
| Lipids | Vegetable Oil |
| Proteins | Beef |
| Nucleic Acids | DNA |
Carbohydrates

Glucose - A carbohydrate that is beneficial to plants
- What is a carbohydrate?
- Compounds made up of carbon, hydrogen, and oxygen atoms. These are usually combined in a ratio of 1, 2, 1.
- Why are they important in living things?
- Short-Term Energy Use and carbohydrates serve as a structure in organisms... EX: Chitin in exoskeleton of athropods.
- What are monomers for carbohydrates known as?
- Monosaccharides
- What are the three monosaccharides for carbohydrates?
 Glucose
Glucose Galactose
Galactose Fructose
Fructose
- Monosaccharides bond together to form chains of polysaccharides.
- EX: Glycogen1, Cellulose2, Chitin3
How much energy is in 1 gram of carbohydrates? 4 CALORIES
References
- Glycogen is a carbohydrate storage in animals.
- Cellulose is a carbohydrate in cell walls of plants.
- Chitin is a carbohydrate in the cell walls of bacteria and fungi
Lipids
- What are lipids?
- Macromolecules that are generally not soluble in water. They are composed of carbon, hydrogen, and oxygen.
- What makes up a lipid monomer?
- Glycerol and Fatty Acid Chains
Importance of Lipids

Crocodile Oil
- Long-term energy storage
- Protection/Insulation
- Membrane Structure
- Acting as a chemical messenger
- Lipid Polymers
- Fats - Come from animals and is solid at room temperature.
- Oils - Come from plants and stays liquid at room temperature.
- Waxes - Come from bees.
Satured and Unsaturated Fatty Acids
1. What is a saturated fatty acid?
- When there are only single bonds between all carbon atoms in the fatty acid chains of a lipid.
2. What is an unsaturated fatty acid?
- When there are double and triple bonds between carbon atoms in a fatty acid chain.
How much energy in 1 gram of lipid? 9 CALORIES

Look at the bonds in the saturated and unsaturated fatty acids
Nucleic Acids
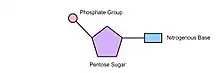
A nucleotide
- Nucleic acids are macromolecules that contain the following elements
- Carbon
- Hydrogen
- Oxygen
- Nitrogen
- Phosphorus
- The monomers for nucleic acids are called...
- Nucleotides
- Three components of a nucleic acid nucleotide are
- Phosphate group
- 5-carbon sugar
- Nitrogenous Base
- Nucleotides will bond together to form...
- Nucleic Acids
- The main function of nucleic acids is to...
- Store and transmit genetic information

DNA
- Two kinds of nucleic acids are
- DNA
- RNA
Proteins

Take note: Amino group on the left, Carboxyl group on the right, and the special "R" group
- Proteins are macromolecules that contain the following elements:
- Carbon
- Hydrogen
- Oxygen
- Nitrogen
- The monomers for proteins are called amino acids.
- The general structure of an amino acid is:
- All amino acids have an amino group and a carboxyl group
- The R group distinguishes one amino acid from another
- There are a total of 20 amino acids
- Amino acids are bonded together through peptide bonds to form protein--or polypeptide chains.
Organizations

Proteins are joined together in up to four different levels of organization.
Primary
- Polypeptidie chain of amino acids.
Secondary
- Polypeptide chain can twist (helix) or fold (sheets) due to weak bonds between amino acids.
Tertiary
- Polypeptide chain as whole twists and folds.
Quaternary
- Multiple chains are arranged into a complex protein (2-4 polypeptide chains grouped together).
Functions
- Structural components in cells
- Regulate cell processes and chemical reactions
- Transport subsbtances across the cell membrane
Overview

This article is issued from Wikiversity. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.