Sulfurous acid
Sulfurous acid is a weak acid with the chemical formula H2SO3. It is produced by dissolving sulfur dioxide in water. Bases deprotonate (remove the Hydrogen ion) it to produce sulfites. It tends to turn back into sulfur dioxide and water again. It is a weak reducing agent.
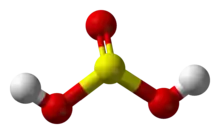
Sulfurous acid chemical structure
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.