Phosphorus(III) oxide
Phosphorus(III) oxide, also known as phosphorus trioxide, is a chemical compound. Its chemical formula is P2O3 or P4O6. It contains phosphorus in its +3 oxidation state. It contains phosphorus and oxide ions.
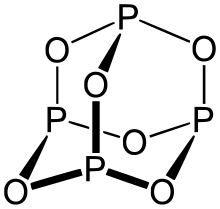
Phosphorus trioxide structure
Properties
It is a white, waxy solid that can absorb water. It reacts with water to make phosphorous acid. It reacts with hydrochloric acid to make phosphorus trichloride and phosphorous acid. It is a reducing agent. It is toxic and has a garlic-like odor.
Preparation
It is made by burning phosphorus in a little air. This is the source of many phosphorus compounds.
Uses
It can attach to certain transition metals.
Related pages
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.