Solvation
Solvation, commonly called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute. If the molecules or ions of the solute are more strongly attracted to the molecules of the solvent than to each other, solvation can occur.
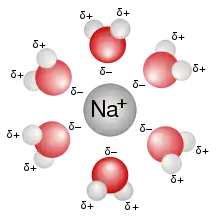
A sodium ion solvated by water molecules
The solvation is the action of dissolving the mix to form the solution.
Related pages
More reading
- Dogonadze, Revaz R. (1985). The Chemical Physics of Solvation. Amsterdam: Elsevier. ISBN 0-444-42551-9 (part A), ISBN 0-444-42674-4 (part B), ISBN 0-444-42984-0 (part C)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.