 | |
| Names | |
|---|---|
| IUPAC name
Zinc(II) fluoride | |
| Other names
Zinc difluoride | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.092 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 3077 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| ZnF2 | |
| Molar mass | 103.406 g/mol (anhydrous) 175.45 g/mol (tetrahydrate) |
| Appearance | white needles hygroscopic |
| Density | 4.95 g/cm3 (anhydrous) 2.30 g/cm3 (tetrahydrate) |
| Melting point | 872 °C (1,602 °F; 1,145 K) (anhydrous) 100 °C, decomposes (tetrahydrate) |
| Boiling point | 1,500 °C (2,730 °F; 1,770 K) (anhydrous) |
| .000052 g/(100 mL) (anhydrous) 1.52 g/(100 mL), 20 °C (tetrahydrate) | |
| Solubility | sparingly soluble in HCl, HNO3, ammonia |
| −38.2·10−6 cm3/mol | |
| Structure | |
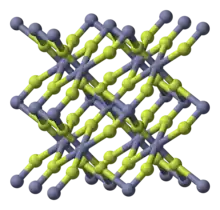
| tetragonal (anhydrous), tP6 | |
| P42/mnm, No. 136 | |
| Hazards | |
| GHS labelling:[1] | |
   | |
| Danger | |
| H301, H315, H318, H335 | |
| P261, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P310, P312, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
|
Other cations |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Zinc fluoride is an inorganic chemical compound with the chemical formula ZnF2. It is encountered as the anhydrous form and also as the tetrahydrate, ZnF2·4H2O (rhombohedral crystal structure).[2] It has a high melting point and has the rutile structure containing 6 coordinate zinc, which suggests appreciable ionic character in its chemical bonding.[3] Unlike the other zinc halides, ZnCl2, ZnBr2 and ZnI2, it is not very soluble in water.[3]
Like some other metal difluorides, ZnF2 crystallizes in the rutile structure, which features octahedral Zn cations and trigonal planar fluorides.[4]
Preparation and reactions
Zinc fluoride can be synthesized several ways.
- The reaction of zinc metal with fluorine gas.[3]
- Reaction of hydrofluoric acid with zinc, to yield hydrogen gas (H2) and zinc fluoride (ZnF2).[3]
Zinc fluoride can be hydrolysed by hot water to form the zinc hydroxide fluoride, Zn(OH)F.[5]
The salt is believed to form both a tetrahydrate and a dihydrate.[6]
References
- ↑ "ZINC fluoride". pubchem.ncbi.nlm.nih.gov.
- ↑ Perry, D. L.; Phillips, S. L. (1995). Handbook of Inorganic Compounds. CRC Press. ISBN 0-8493-8671-3.
- 1 2 3 4 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Stout, J. W.; Reed, Stanley A. (1954). "The Crystal Structure of MnF2, FeF2, CoF2, NiF2 and ZnF2". J. Am. Chem. Soc. 76 (21): 5279–5281. doi:10.1021/ja01650a005.
- ↑ Srivastava, O. K.; Secco, E. A. (1967). "Studies on Metal Hydroxy Compounds. I. Thermal Analyses of Zinc Derivatives ε-Zn(OH)2, Zn5(OH)8Cl2 · H2O, β-ZnOHCl, and ZnOHF". Canadian Journal of Chemistry. 45 (6): 579–583. doi:10.1139/v67-096.
- ↑ Lindahl, Charles B.; Mahmood, Tariq (2000), "Fluorine compounds, inorganic, zinc", Kirk-Othmer Encyclopedia of Chemical Technology, New York: John Wiley, doi:10.1002/0471238961.2609140312091404.a01, ISBN 9780471238966
