 | |
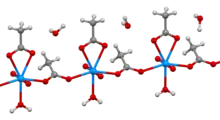 Hydrated crystal structure | |
| Names | |
|---|---|
| IUPAC name
Uranium bis((acetato)-O)dioxo-dihydrate | |
| Other names
Uranyl ethanoate; Uranyl acetate dihydrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.971 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| UO2(CH3COO)2 (anhydrous) UO2(CH3COO)2·2H2O (dihydrate) | |
| Molar mass | 424.146 g/mol (dihydrate) |
| Appearance | yellow-green crystals (dihydrate) |
| Density | 2.89 g/cm3 (dihydrate) |
| Melting point | decomposes at 80 °C (dihydrate) |
| 7-8 g/100 ml | |
| Solubility | slightly soluble in ethanol[1] |
| Hazards | |
| GHS labelling: | |
 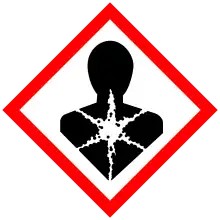 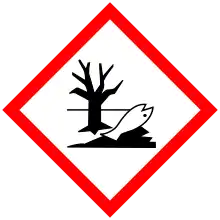 | |
| Danger | |
| H300, H330, H373, H411 | |
| P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P314, P320, P321, P330, P391, P403+P233, P405, P501 | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uranyl acetate is the acetate salt of uranium oxide, a toxic yellow-green powder useful in certain laboratory tests. Structurally, it is a coordination polymer with formula UO2(CH3CO2)2(H2O)·H2O.
Structure
In the polymer, uranyl (UO22+) centers are bridged by acetate ligands. The remainder of each (heptacoordinate) coordination sphere is provided by an aquo ligand and a bidentate acetate ligand. One water of crystallization occupies the lattice.[2]
Uses
Uranyl acetate is extensively used as a negative stain in electron microscopy.[3] Most procedures in electron microscopy for biology require the use of uranyl acetate. Negative staining protocols typically treat the sample with 1% to 5% aqueous solution. Uranyl acetate staining is simple and quick to perform and one can examine the sample within a few minutes after staining. Some biological samples are not amenable to uranyl acetate staining and, in these cases, alternative staining techniques and or low-voltage electron microscopy technique may be more suitable.
1% and 2% uranyl acetate solutions are used as an indicator, and a titrant in stronger concentrations in analytical chemistry, as it forms an insoluble salt with sodium (the vast majority of sodium salts are water-soluble). Uranyl acetate solutions show evidence of being sensitive to light, especially UV, and will precipitate if exposed.
Uranyl acetate is also used in a standard test—American Association of State Highway and Transportation Officials (AASHTO) Designation T 299—for alkali-silica reactivity in aggregates (crushed stone or gravel) being considered for use in cement concrete.
Uranyl acetate dihydrate has been used as a starting reagent in experimental inorganic chemistry.[4]
Related compounds
Uranyl carboxylates are known for diverse carboxylic acids (formate, butyrate, acrylate).[5]
Safety
Uranyl acetate is both chemically toxic and mildly radioactive. Chronic-exposure effects may cumulate.
In general, uranium salts exhibit nephrotoxicity. Normal commercial stocks from depleted uranium have typical specific activity 0.37–0.51 microcuries per gram (14–19 kBq/g), too weak to harm from outside the body. However, uranyl acetate is very toxic if ingested, inhaled as dust, or absorbed through cut or abraded skin.
Microbiologists have developed a number of alternative stains:[6] neodymium acetate,[7][8] platinum blue,[9] hafnium chloride,[10] and oolong tea extracts.[11][12]
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–566, ISBN 0-8493-0594-2
- ↑ Howatson, J.; Grev, D.M.; Morosin, B. (1975). "Crystal and molecular structure of uranyl acetate dihydrate". Journal of Inorganic and Nuclear Chemistry. 37 (9): 1933–1935. doi:10.1016/0022-1902(75)80918-3.
- ↑ "Negative Staining" University of Oxford
- ↑ Sessler, Jonathan L.; Seidel, Daniel; Vivian, Anne E.; Lynch, Vincent; Scott, Brian L.; Keogh, D. Webster (2001). "Hexaphyrin(1.0.1.0.0.0): An Expanded Porphyrin Ligand for the Actinide Cations Uranyl (UO22+) and Neptunyl (NpO2+)". Angewandte Chemie International Edition. 40 (3): 591–594. doi:10.1002/1521-3773(20010202)40:3<591::AID-ANIE591>3.0.CO;2-0.
- ↑ Klepov, Vladislav V.; Vologzhanina, Anna V.; Alekseev, Evgeny V.; Pushkin, Denis V.; Serezhkina, Larisa B.; Sergeeva, Olga A.; Knyazev, Aleksandr V.; Serezhkin, Viktor N. (2016). "Structural diversity of uranyl acrylates". CrystEngComm. 18 (10): 1723–1731. doi:10.1039/C5CE01957E.
- ↑ Yamaguchi K, Suzuki K, Tanaka K (2010) Examination of electron stains as a substitute for uranyl acetate for the ultrathin sections of bacterial cells. J Electron Microsc (Tokyo) 59:113–118
- ↑ Kuipers, Jeroen; Giepmans, Ben N. G. (1 April 2020). "Neodymium as an alternative contrast for uranium in electron microscopy". Histochemistry and Cell Biology. 153 (4): 271–277. doi:10.1007/s00418-020-01846-0. ISSN 1432-119X. PMC 7160090. PMID 32008069.
- ↑ Hosogi N, Nishioka H, Nakakoshi M (2015) Evaluation of lanthanide salts as alternative stains to uranyl acetate. Microscopy (Oxf) 64:429–435
- ↑ Inaga S, Katsumoto T, Tanaka K, Kameie T, Nakane H, Naguro T (2007) Platinum blue as an alternative to uranyl acetate for staining in transmission electron microscopy. Arch Histol Cytol 70:43–49
- ↑ Ikeda K, Inoue K, Kanematsu S, Horiuchi Y, Park P (2011) Enhanced effects of nonisotopic hafnium chloride in methanol as a substitute for uranyl acetate in TEM contrast of ultrastructure of fungal and plant cells. Microsc Res Tech 74:825–830
- ↑ Sato S, Adachi A, Sasaki Y, Ghazizadeh M (2008) Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections. J Microsc 229:17–20
- ↑ He X, Liu B (2017) Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections based on examples of animal tissues for transmission electron microscopy. J Microsc 267:27–33